Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Limited information exists on sex-related differences in axial spondyloarthritis (axSpA) randomized controlled trials (RCTs). Through a systematic literature review and meta-analysis we aimed to assess sex-related differences in patient characteristics and efficacy and safety end-points of advanced therapies in axSpA RCTs.
Methods: We performed a systematic literature search in MEDLINE, EMBASE, Central and FDA databases, from January 2000 to March 19, 2023. We included RCTs that assessed the efficacy of advanced therapies in adult participants with axSpA. Two reviewers extracted information on the rates of the following end-points by sex: Assessment in Spondylarthritis International Society criteria (ASAS40/20), and the Ankylosing Spondylitis Disease Activity Score low disease activity/inactive disease (ASDAS-LDA/ID; ASDAS< 2.1). We used random-effects models to calculate pooled effects for responses in males vs. females for the different classes of advanced therapies (Odds ratio (OR) and 95% Confidence interval (CI)).
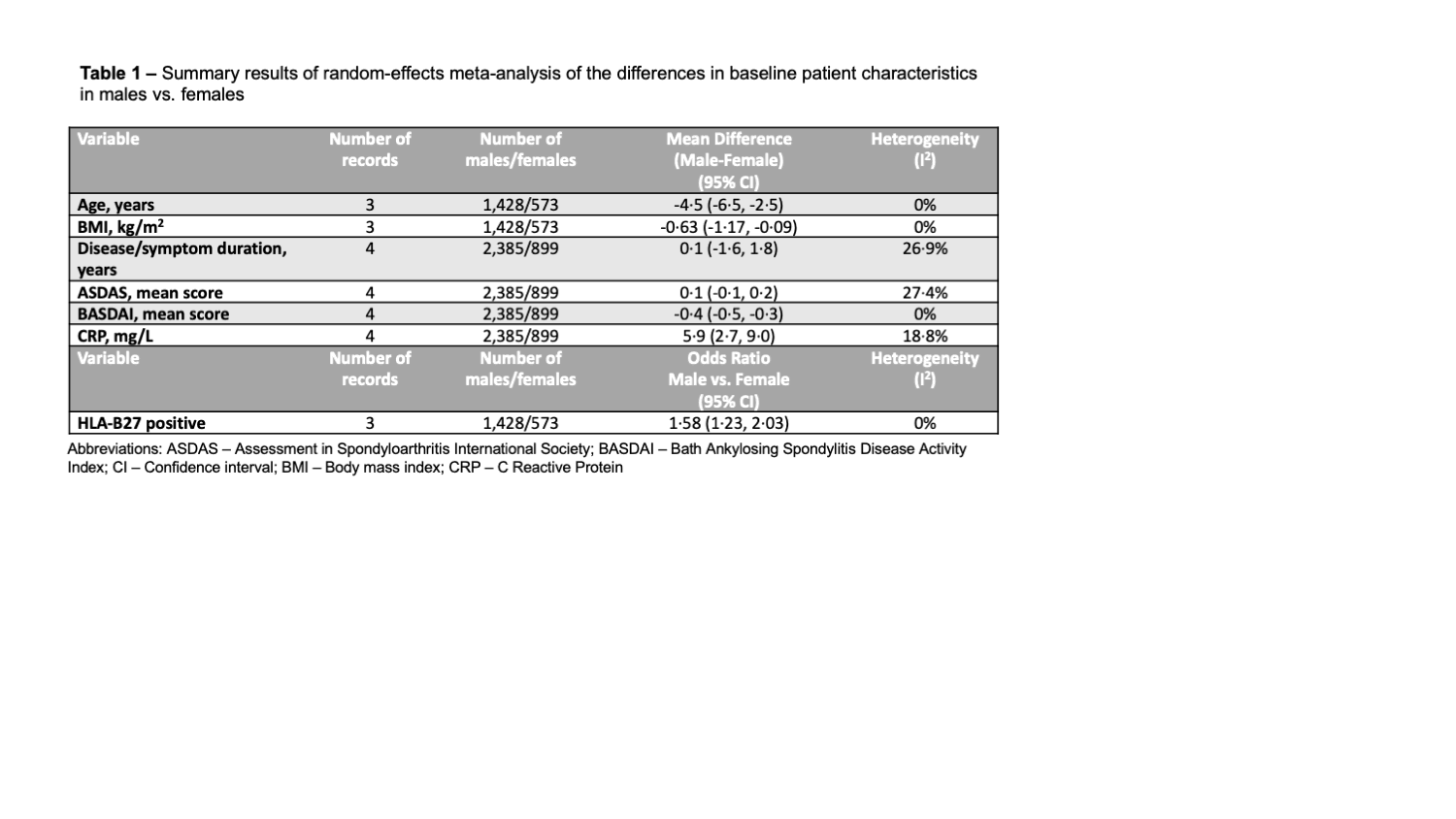
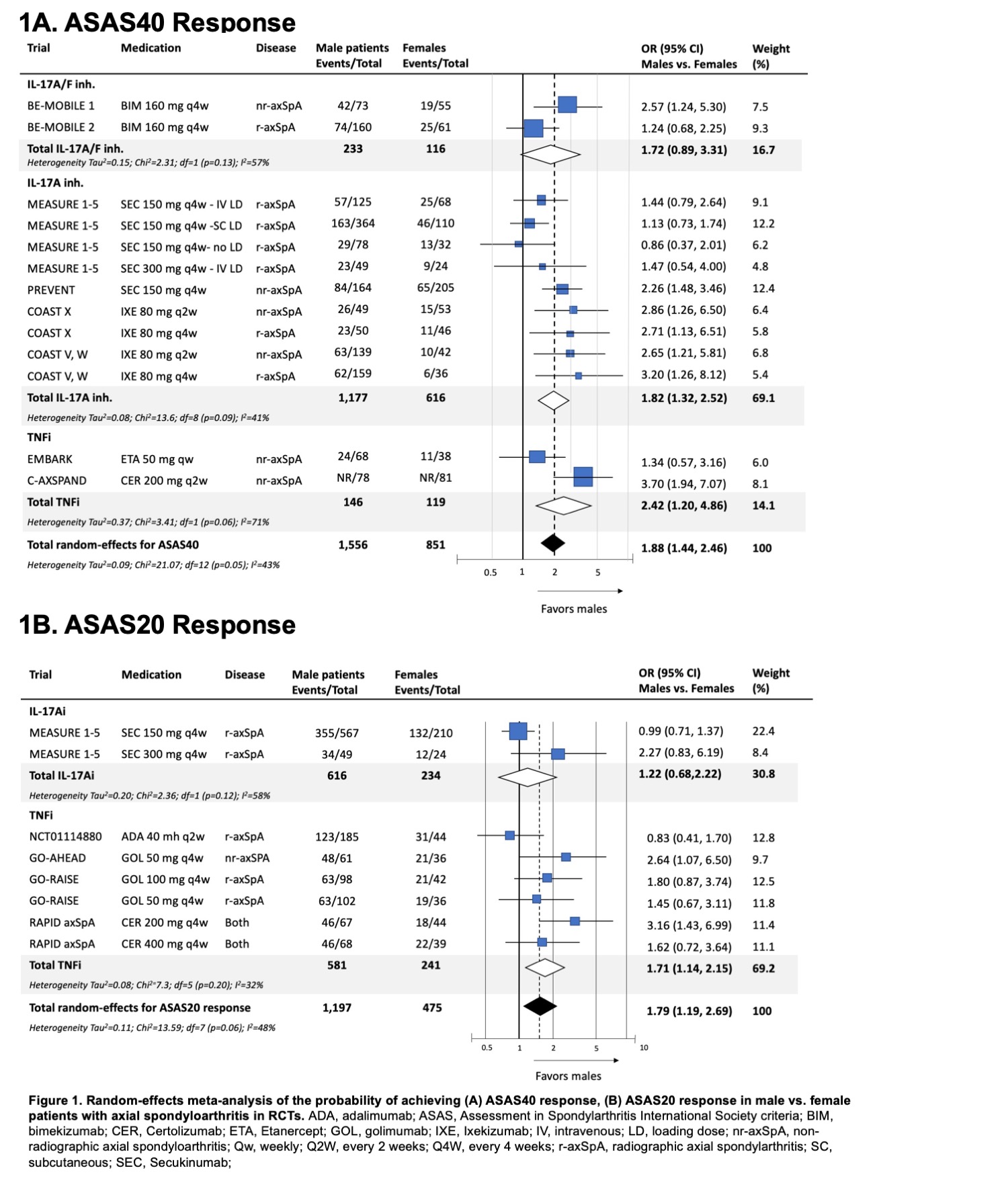
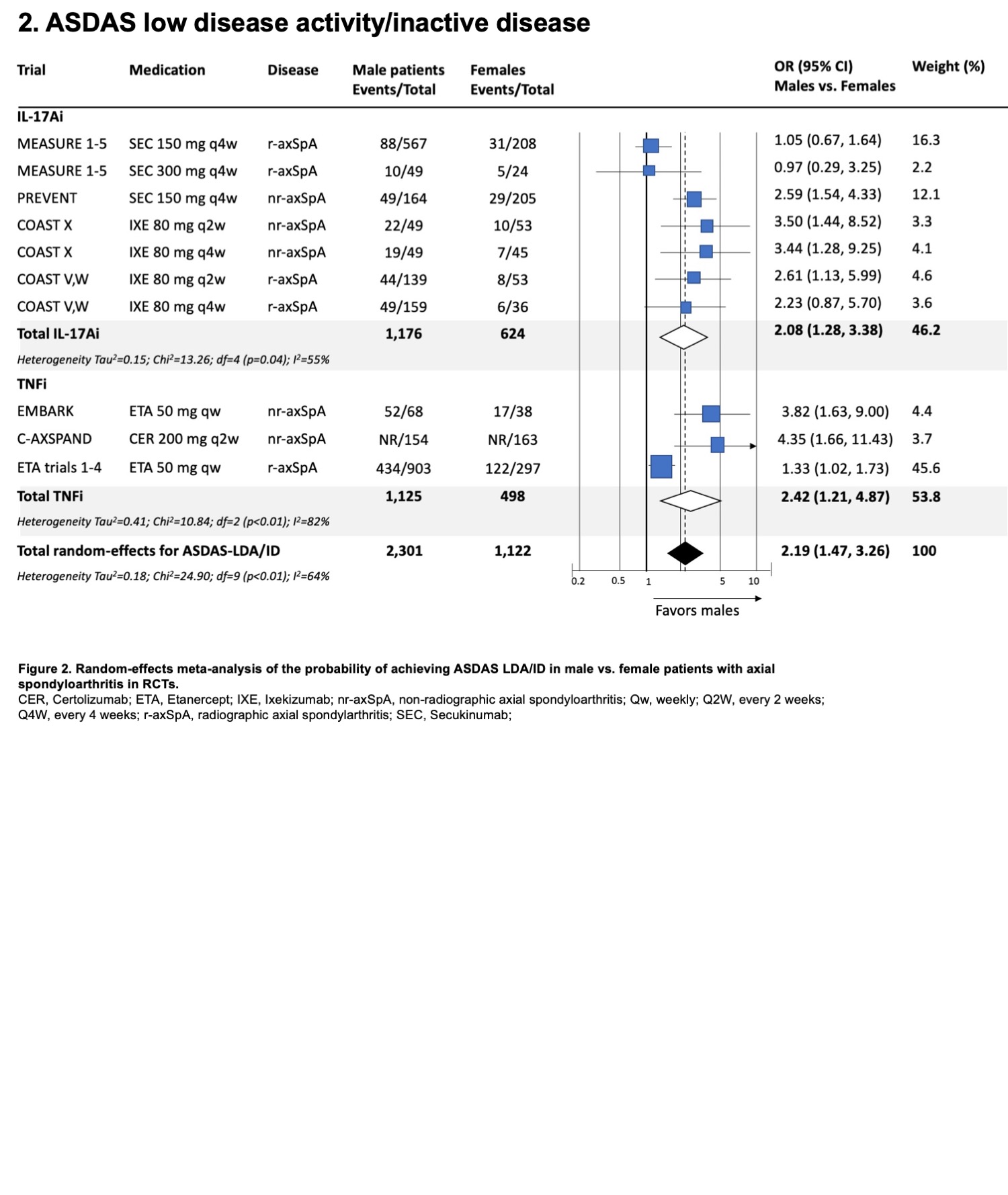
Results: A total of 79 RCTs were included (23,748 participants). The ratio of male to female was around 2:1 (69.7% male participants). Only 9 trials (11.4%) reported sex-disaggregated baseline characteristics, 22 trials (27.8%) reported sex-disaggregated efficacy endpoints and 9 trials (11.4%) reported sex-disaggregated safety endpoints. Female patients were significantly older, had higher baseline BASDAI scores, and higher body mass index (Table 1). Male patients had significantly higher baseline C-reactive protein and were more likely to be HLA-B27 positive. Overall, male patients on advanced therapies were more likely to achieve an ASAS40 response compared to female patients (OR 1.88, 95% CI 1.44, 2.46; Figure 1A). This was significant for both IL-17A inhibitors (i) (OR 1.82) and TNFi (OR 2.42) and numerically higher for IL-17A/Fi (OR 1.72). Male patients were also more likely to achieve ASAS20 response (OR 1.79, 95% CI 1.19, 2.69; Figure 1B) and ASDAS-ID or LDA response (OR 2.19, 95% CI 1.47, 3.26; Figure 2). Subgroup analyses and meta-regression showed that heterogeneity in effect size was influenced primarily by the underlying SpA feature, specifically r-axSpA vs. nr-axSpA. The odds of achieving efficacy endpoints among males were notably higher in nr-axSpA patients (OR 2.60 to 3.76) compared to those with r-axSpA (OR 1.21 to 1.72). No significant differences in effect size were noted across drug classes or bio-exposure status. ASAS40 placebo response was not significantly different across sexes (OR 1.35, 95% CI 0.94, 1.92).
Conclusion: Female patients participating in RCTs are less likely to achieve efficacy outcomes than their male counterparts. This trend was similar across classes of biologic therapies.
CER, Certolizumab; ETA, Etanercept; IXE, Ixekizumab; nr-axSpA, non-radiographic axial spondyloarthritis; Qw, weekly; Q2W, every 2 weeks; Q4W, every 4 weeks; r-axSpA, radiographic axial spondylarthritis; SEC, Secukinumab;
To cite this abstract in AMA style:
Gao A, Pardo Pardo J, Dang S, Gensler L, Mease P, Eder L. Sex-related Differences in Efficacy and Safety Outcomes in Axial Spondyloarthritis Randomized Clinical Trials: A Systematic Literature Review and Meta-analysis [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/sex-related-differences-in-efficacy-and-safety-outcomes-in-axial-spondyloarthritis-randomized-clinical-trials-a-systematic-literature-review-and-meta-analysis/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/sex-related-differences-in-efficacy-and-safety-outcomes-in-axial-spondyloarthritis-randomized-clinical-trials-a-systematic-literature-review-and-meta-analysis/