Session Information
Date: Monday, October 27, 2025
Title: Abstracts: Spondyloarthritis Including Psoriatic Arthritis – Treatment I: Therapies (0873–0878)
Session Type: Abstract Session
Session Time: 10:00AM-10:15AM
Background/Purpose: PsA affects male and female patients equally; however, variations in manifestations and treatment response exist between sexes. Deucravacitinib is an oral, selective, allosteric tyrosine kinase 2 (TYK2) inhibitor. This post hoc pooled analysis of 2 randomized, placebo (PBO)–controlled, phase 3, pivotal studies (POETYK PsA-1 [NCT04908202] and POETYK PsA-2 [NCT04908189]) assessed proteomic profiles in patients with PsA by sex to identify relevant biological pathways and evaluate the effect of deucravacitinib on disease activity–related proteomic scores.
Methods: Proteomic profiling of plasma samples from POETYK PsA-1 and PsA-2 was conducted using Olink 3072, a multiplex assay that includes ≈ 3000 proteins, and harmonized using 24 bridging samples. Differential expression (DE) analysis was conducted using DREAM. Significant DE proteins were identified for all patients vs demographically matched normal healthy volunteers (NHVs; fold change [FC] = 1.5; adj P < 0.05). The FC threshold was 1.23 (adj P < 0.05) for male vs female patient comparisons. Enriched pathways were identified using clusterProfiler based on DE proteins. Disease state protein signatures were derived from baseline DE proteins in all patients vs all NHVs, female patients vs female NHVs, and male patients vs male NHVs. Disease activity protein signatures were independently derived by selecting proteins with > 0.3 repeated measure correlations with clinical endpoints. Protein signature scores were calculated using z score normalization to standardize expression across samples. The geometric mean of the z scores for constituent proteins was calculated to obtain the final signature score via the Gene Set Variation Analysis package.
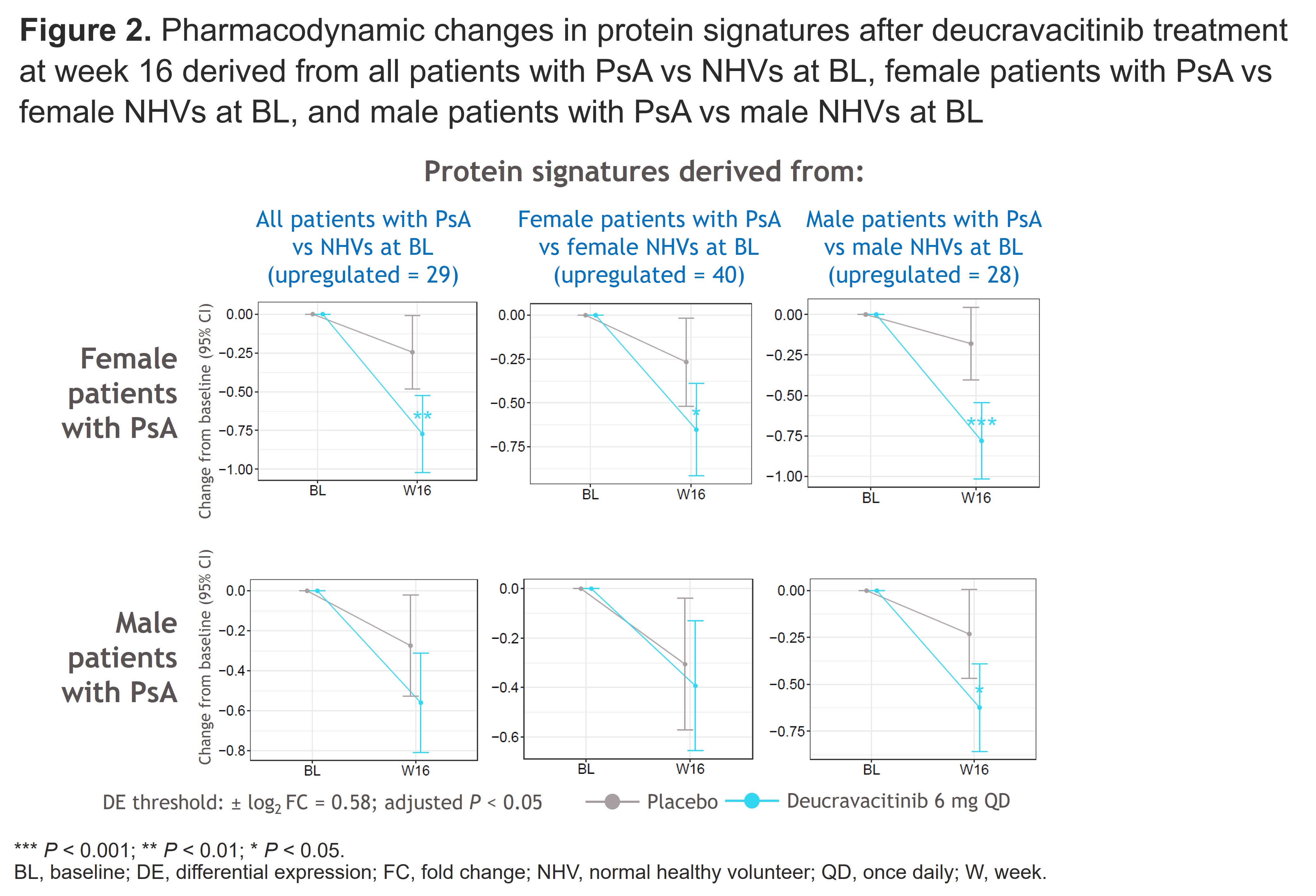
Results: DE proteins were seen in patients with PsA (n = 1222) vs NHVs (n = 121) in a KEGG pathway analysis (Figure 1A). Upregulated proteins (eg, PI3, IL-17, IL-19, beta-defensin, MMP3) were reduced with deucravacitinib vs PBO (Figure 1B). Overall, 133 and 177 unique proteins were identified in male and female patients, respectively, vs sex-matched NHVs. Female patients had greater enrichment in neuroactive ligand receptor interaction and hormone metabolism pathways vs male patients. Regardless of whether disease state protein signature scores were derived from all patients, female patients, or male patients, deucravacitinib reduced these scores vs PBO in female and male patients at W16 (Figure 2). Deucravacitinib also significantly reduced protein signature scores associated with PsA-relevant disease activity vs PBO in male and female patients.
Conclusion: DE was identified in patients with PsA vs NHVs. Deucravacitinib reduced upregulated proteins in all patients. Unique proteomic profiles were identified in male vs female patients with PsA. Deucravacitinib reduced disease state signature scores in the overall population, male patients, and female patients, which is consistent with improved clinical efficacy in the overall population and by sex. Deucravacitinib also reduced disease activity signature scores in male and female patients. Findings provide insight into sex-specific PsA disease biology and may help personalize disease management.
To cite this abstract in AMA style:
Eder L, Li S, Mease P, Ritchlin C, Maksymowych W, Schulze-Koops H, Smolen J, FitzGerald O, Chandran V, Wu C, Liu J. Sex Differences in Proteomic Profiles and the Impact of Deucravacitinib Treatment in Patients with Active Psoriatic Arthritis: A Pooled Phase 3 Analysis [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/sex-differences-in-proteomic-profiles-and-the-impact-of-deucravacitinib-treatment-in-patients-with-active-psoriatic-arthritis-a-pooled-phase-3-analysis/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/sex-differences-in-proteomic-profiles-and-the-impact-of-deucravacitinib-treatment-in-patients-with-active-psoriatic-arthritis-a-pooled-phase-3-analysis/

.jpg)