Session Information
Date: Tuesday, November 14, 2023
Title: (2019–2038) Patient Outcomes, Preferences, & Attitudes Poster III
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
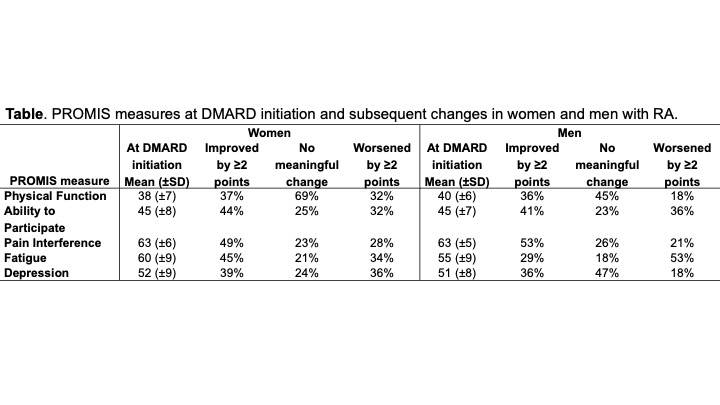
Background/Purpose: Patient-Reported Outcome Measurement Information System (PROMIS®) measures are valued in the assessment of health outcomes for patients with rheumatoid arthritis (RA). However, little is known about sex differences in change in key PROMIS domains following initiation of DMARD therapy. We aimed to examine sex differences in PROs among patients with RA after starting a new DMARD.
Methods: In this retrospective study, patients with RA seen at a single institution between 1/2020 and 10/2021 were included. RA patients were identified by presence of 2 diagnostic codes (ICD-10: M05.x/M06.x, excluding M06.1) and prescription of a DMARD. PROMIS computer adaptive tests for Physical Function, Pain Interference, Fatigue, Depression, and Ability to Participate in Social Roles and Activities were routinely collected at each clinical visit. Patients with available PROMIS measures at the start of a new conventional synthetic (cs), biologic (b), or targeted synthetic (ts) DMARD and at least one subsequent visit were included in the analysis. Minimum clinically important differences (MCID) and meaningful differences were defined for each PROMIS measure (ref: Bartlett et al, AC&R 2022). Generalized binomial models with logit link were used to examine sex differences in improvement or worsening of PROMIS measures over time, adjusting for age, DMARD type and duration.
Results: A total of 179 patients with RA (76% female; 93% white; mean age 56.2 years) who started 202 new DMARDs (85 csDMARDs, 88 bDMARDs and 29 tsDMARDs) were studied. Median follow-up was 5.8 (interquartile range: 3.7-9.4) months per DMARD. Mean (±SD) PROMIS measures at DMARD initiation were 38.7 (±7.0) for physical function, 44.7 (±7.8) for social participation, 62.7 (±5.8) for Pain interference, 58.6 (±9.2) for fatigue and 5.20 (±8.7) for depression. Women were 3 times more likely to have worsening of their depression score by at least 2 points after starting a new DMARD (36% of women worsened vs 18% of men; odds ratio: 3.07; 95% CI: 1.29-7.30; Table). This association persisted after adjusting for change in physical function (OR: 2.86; 95% CI: 1.16-7.01). This association also persisted (but did not reach statistical significance) in the subset of patients who experienced a meaningful improvement in physical function (OR: 4.16; 95% CI: 0.71-24.44). No statistically significant sex differences were noted in pain interference (p=0.69) or ability to participate (p=0.23). Women were somewhat more likely to experience worsening physical function (OR: 2.24; 95% CI: 0.92-5.48; p=0.08) and were somewhat less likely to experience worsening fatigue (OR: 0.56; 95% CI: 0.26-1.21; p=0.14) compared to men, but these associations did not reach statistical significance.
Conclusion: We observed that women with RA initiating a new DMARD were more likely to experience worsening depression as compared to men, even when they experienced treatment-associated improvement in physical function. The findings point to important sex differences in PRO responses to DMARD therapy and highlight the value of PROMIS measures in understanding sex differences in RA health outcomes.
To cite this abstract in AMA style:
Davis J, Achenbach S, Arment C, Bekele D, Kronzer V, Mason T, Myasoedova E, Peterson L, Wright K, Crowson C. Sex Differences in Patient-Reported Outcomes in Patients with Rheumatoid Arthritis After Starting a New Disease-Modifying Antirheumatic Medication [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/sex-differences-in-patient-reported-outcomes-in-patients-with-rheumatoid-arthritis-after-starting-a-new-disease-modifying-antirheumatic-medication/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/sex-differences-in-patient-reported-outcomes-in-patients-with-rheumatoid-arthritis-after-starting-a-new-disease-modifying-antirheumatic-medication/