Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Children have the lowest COVID-19 vaccination rates of any age group. In adults with rheumatic diseases, barriers to vaccination include the perceived lack of vaccine testing in this patient group and concerns for safety among immunocompromised individuals. Whether parents of children with pediatric rheumatic disease (PRD) share these hesitations remains unknown, as adverse events of COVID-19 vaccination have not been well described in this patient population. In addition, there is limited understanding of the severe and long-term complications of COVID-19 among children with PRD, including multisystem inflammatory syndrome in children (MIS-C) and post-acute sequelae of COVID-19 (PASC). To address these gaps, we report real-world data from a multilingual international survey of caregivers of children with PRDs, the 2023 COVID-19 Global Rheumatology Alliance Pediatric Survey.
Methods: The survey was written in English and translated into 25 languages. The survey was launched on a Qualtrics server and disseminated globally through email, social media, and partnerships with patient support organizations. Eligible respondents were ≥18 years of age and the caregiver of a child with PRD < 18 years of age. The survey captured caregiver-reported data on severe and long-term COVID-19 outcomes, as well as caregiver vaccine hesitancy and vaccine adverse events among children with PRD. PASC was defined as symptoms lasting 4 weeks or greater after COVID-19 infection, as per CDC guidelines. Data were collected from May 10 to August 4, 2023.
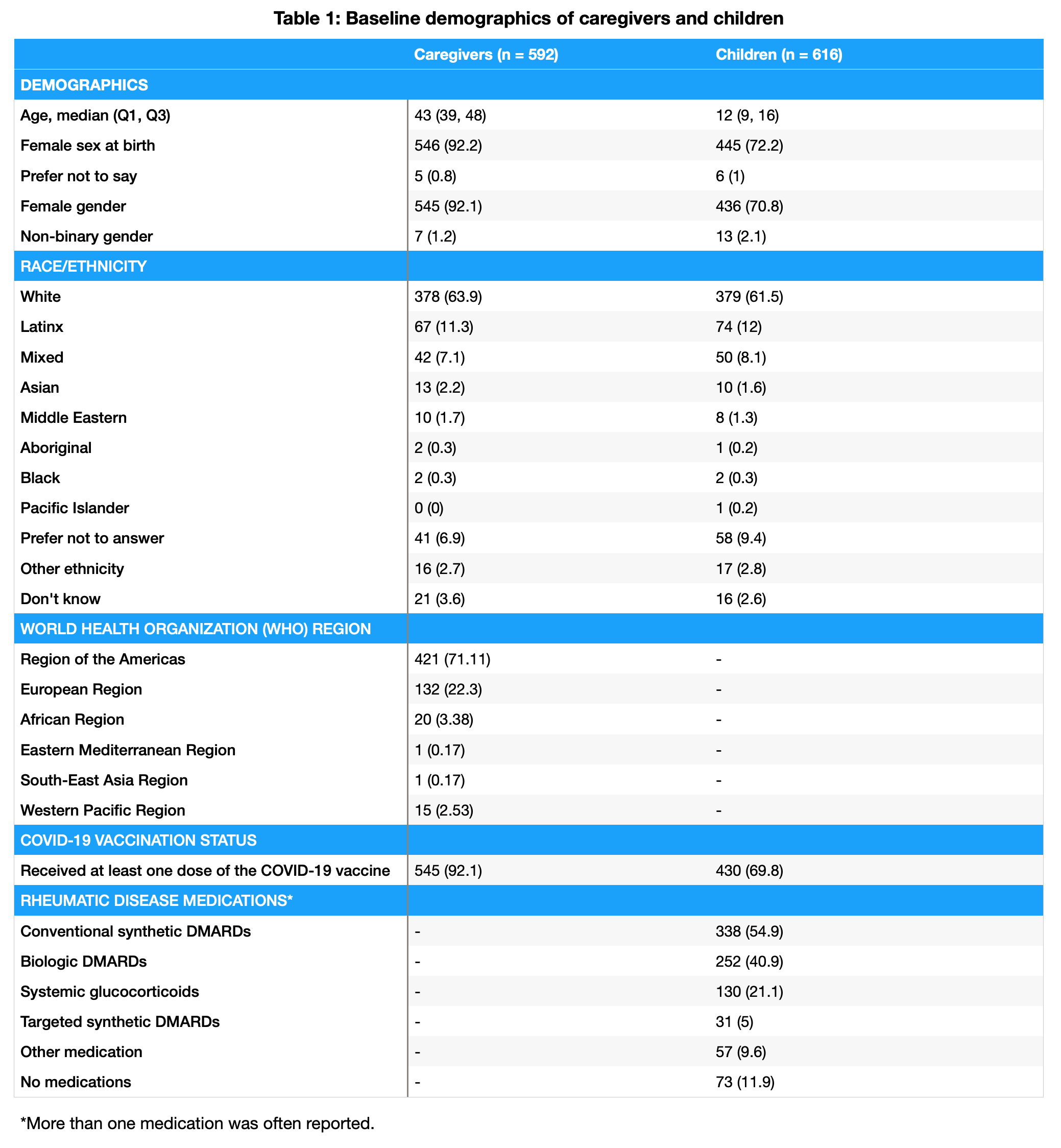
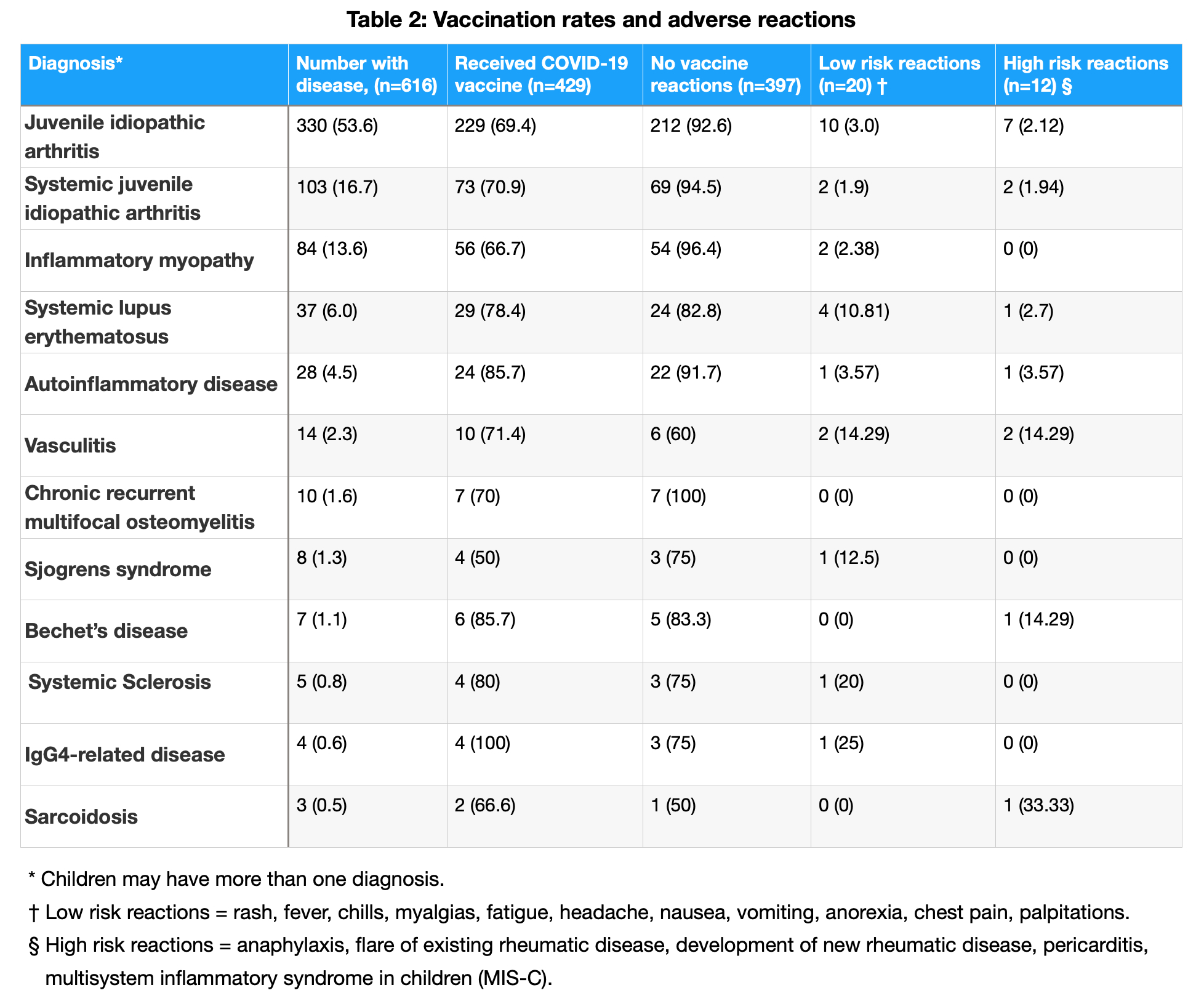
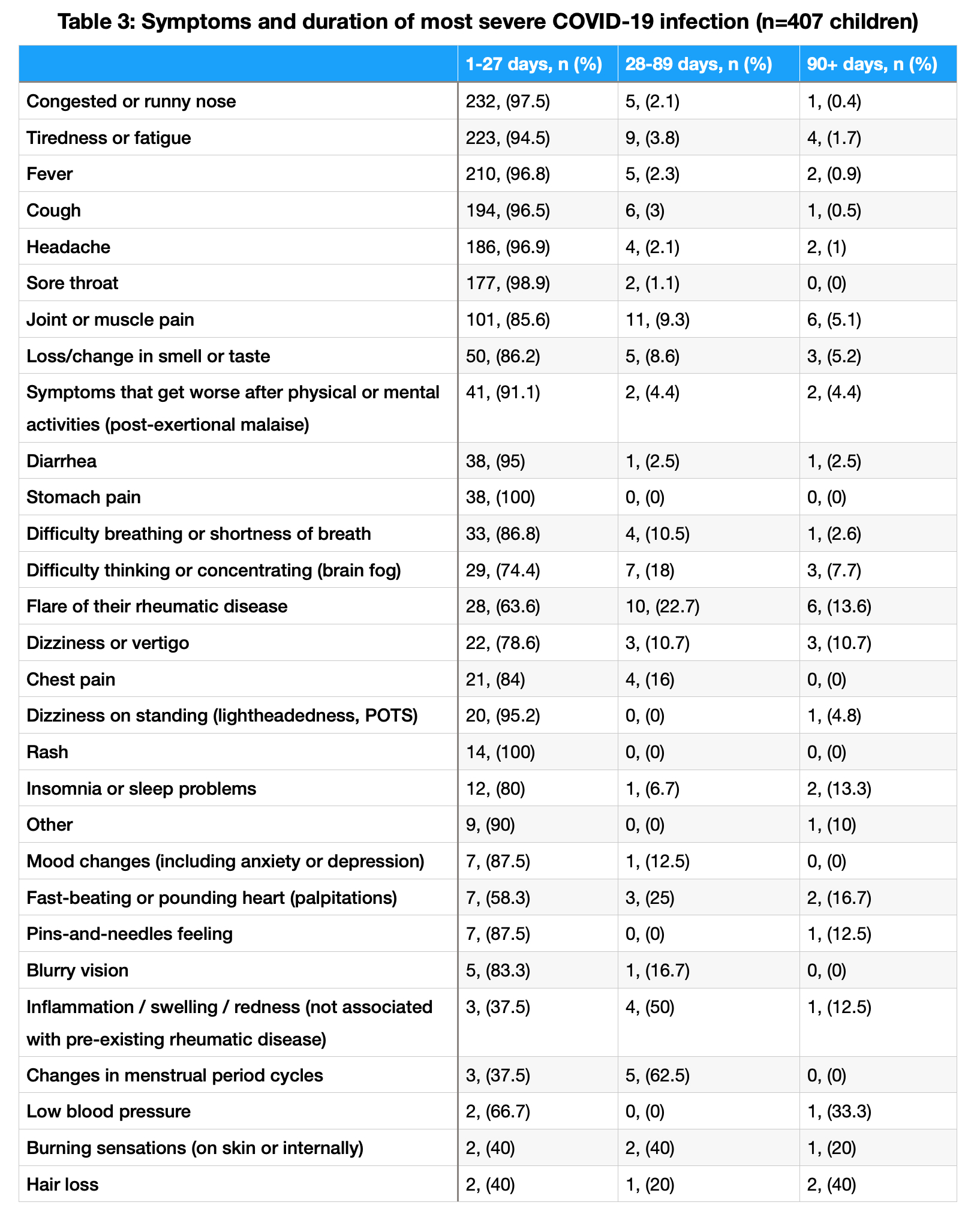
Results: A total of 592 caregivers reported data on 616 children with PRD (Table 1). In caregivers and children, COVID-19 vaccination rates were 92% and 70%, respectively. COVID-19 vaccine adverse events were rare, with severe events occurring in 12 children (2.7%), as shown in Table 2. Among the children, 407 (66%) had experienced at least one COVID-19 infection. Of 552 COVID-19 infections, only 15 children (3.7%) required hospitalization. COVID-19 symptoms and duration are shown in Table 3. While 55 children (13.5%) reported symptoms consistent with PASC, only 32 (58.1%) had been formally diagnosed. MIS-C was reported in 14 children (3.4%); 6 continued having symptoms related to MIS-C at the time of survey completion.
Conclusion: In this global survey of caregivers of children with PRDs, COVID-19 vaccination rates were higher than those reported in otherwise healthy children, and vaccine adverse events were rare. While many children reported COVID-19, very few required hospitalization. A small minority of children reported symptoms consistent with PASC, of which fewer had been formally diagnosed. However, PASC prevalence in this study was lower than in studies of otherwise healthy children, perhaps due to high vaccination rates. Among children with PRD, MIS-C was rare. Limitations of this study include participation bias and the caregiver-reported nature of the survey. Nevertheless, our findings provide real-world data regarding the safety of COVID-19 vaccination among children with PRDs and the infrequency of severe and long-term outcomes of COVID-19 infection.
To cite this abstract in AMA style:
Hausmann J, Kennedy K, Knapp E, Lalonde N, Wallace j, Howard R, Alvarez M, Fabi M, Franco L, Grainger R, Liew J, Machado P, Wallace Z, Yazdany J, Sirotich E. Severe and Long-Term Outcomes of COVID-19 Infection and Vaccine Hesitancy and Adverse Events in Children with Pediatric Rheumatic Diseases: Insights from a COVID-19 Global Rheumatology Alliance Caregiver Survey [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/severe-and-long-term-outcomes-of-covid-19-infection-and-vaccine-hesitancy-and-adverse-events-in-children-with-pediatric-rheumatic-diseases-insights-from-a-covid-19-global-rheumatology-alliance-caregi/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/severe-and-long-term-outcomes-of-covid-19-infection-and-vaccine-hesitancy-and-adverse-events-in-children-with-pediatric-rheumatic-diseases-insights-from-a-covid-19-global-rheumatology-alliance-caregi/