Session Information
Date: Monday, November 18, 2024
Title: Metabolic & Crystal Arthropathies – Basic & Clinical Science Poster III
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Hypertension is the most common comorbidity among patients with gout, with a prevalence of nearly 75% among patients with gout. Losartan and calcium channel blockers (CCB) are commonly prescribed anti-hypertensive medications that have been associated with a lower risk of incident gout. Losartan is specifically endorsed as a preferred anti-hypertensive medication by the ACR gout management guidelines. However, real-world data on whether these medications can lower serum urate among patients with gout is limited. Thus, we sought to examine the serum urate (SU) change among patients treated with losartan or CCB compared to angiotensin converting enzyme inhibitors (ACEi).
Methods: Using the Mass General Brigham electronic health record (EHR) database, we identified all patients with gout and hypertension initiating losartan, CCB, or ACEi who had SU measurements within 3 months before and after medication initiation. Patients with gout were identified using an algorithm with a positive predictive value of 0.9 against the 2015 ACR/EULAR gout classification criteria and required to have baseline SU >6 mg/dL to be included in the analysis. Each EHR was manually reviewed to confirm the gout diagnosis and the initiation and continuation of losartan, CCB, or ACEi during the study period (up to 6 months). Patients who had SU measured < 7 days after the initial prescription or in the setting of an acute gout flare were excluded, as were patients who initiated or change the dose of urate-lowering therapy during the study period. We first calculated the within-group SU change. Then, we compared the SU change between groups (losartan or CCB initiators vs. ACEi initiators) using multivariable linear regression adjusting for relevant covariates including age, sex, baseline SU, baseline ULT use, and comorbidities. We also conducted subgroup analyses according to baseline diuretic use, ULT use, and presence of comorbid chronic kidney disease or type 2 diabetes.
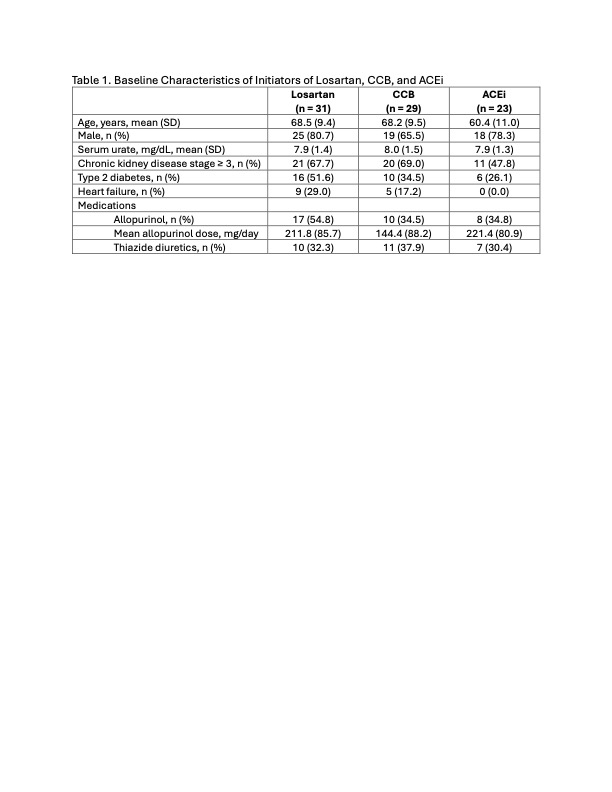
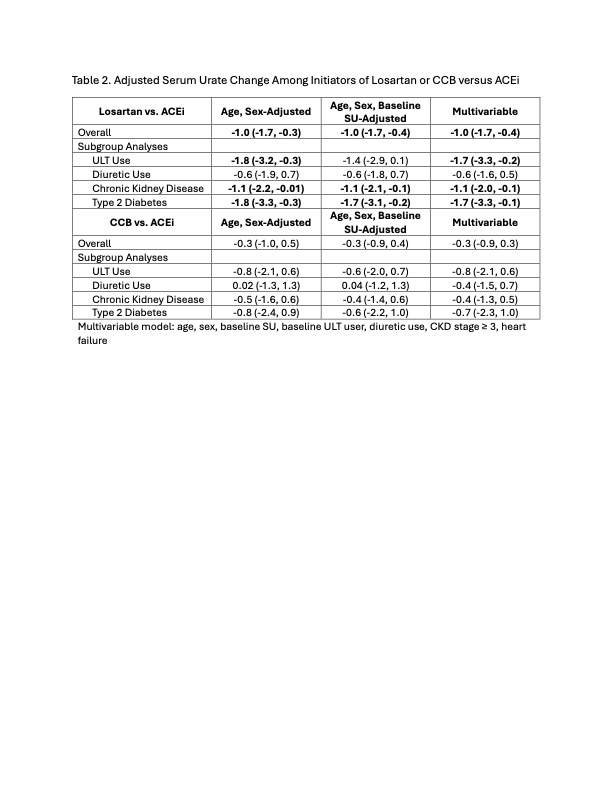
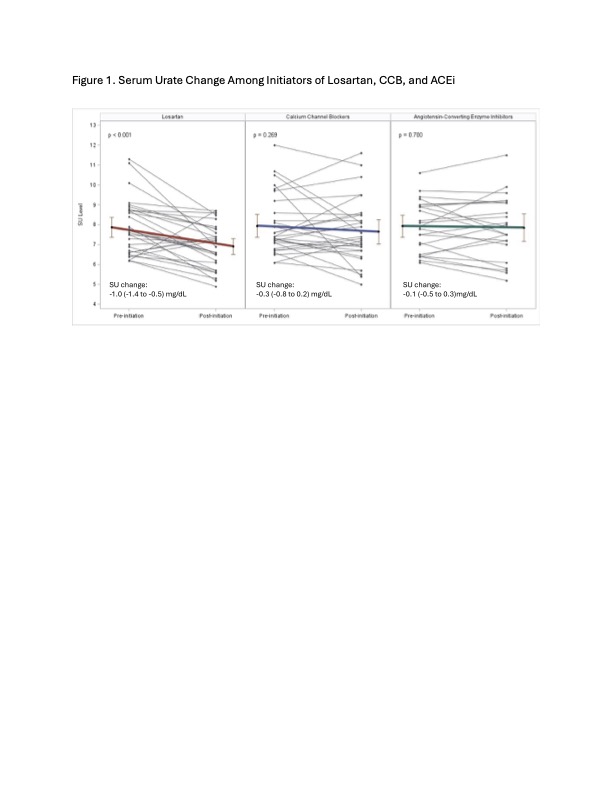
Results: There were 31 patients who started losartan, 29 patients who started CCB, and 23 patients who started ACEi (Table 1). Losartan and CCB initiators tended to be older than ACEi initiators. Mean baseline SU was similar between the three groups, though more losartan initiators tended to be on allopurinol. The mean SU change within losartan initiators was -1.0 (-1.4 to -0.5) mg/dL (p< 0.01), compared to -0.3 (-0.8 to 0.2) mg/dL among CCB initiators (p=0.3) and -0.1 (-0.5 to 0.3) mg/dL among ACEi initiators (p=0.7) (Figure 1). In the linear regression model, losartan initiation was associated with a -1.0 (-1.7 to -0.4) mg/dL greater reduction in SU compared with ACEi; this association remained significant regardless of ULT use or presence of chronic kidney disease or type 2 diabetes (Table 2). CCB initiation was not associated with a greater SU reduction compared to ACEi (Table 2).
Conclusion: In this comparative effectiveness analysis of patients with gout and hypertension and baseline SU >6 mg/dL, losartan initiation was associated with a modest reduction in SU over several months, whereas CCB and ACEi initiation was not. This provides additional evidence supporting use of losartan as the preferred anti-hypertensive option among patients with gout and hyperuricemia.
To cite this abstract in AMA style:
Yokose C, chigurupati s, Jiang B, Tan K, McCormick N, Choi H. Serum Urate Change Among Patients with Gout Treated with Anti-Hypertensive Medications: A Comparative Effectiveness Analysis [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/serum-urate-change-among-patients-with-gout-treated-with-anti-hypertensive-medications-a-comparative-effectiveness-analysis/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/serum-urate-change-among-patients-with-gout-treated-with-anti-hypertensive-medications-a-comparative-effectiveness-analysis/