Session Information
Date: Monday, November 9, 2020
Title: SLE – Treatment Poster II
Session Type: Poster Session D
Session Time: 9:00AM-11:00AM
Background/Purpose: The ALLURE study compared efficacy and safety of abatacept (ABA) vs placebo (PBO) on background MMF and CS for the treatment of active proliferative LN.1 The primary endpoint of improvement in complete response (CR) rate at 1 year (ABA 35.1%, PBO 33.5%; p=0.73) was not met; however, the ABA-treated patient (pt) group had an earlier, more pronounced improvement in urine protein/creatinine ratio (UPCR; ABA –2.95, PBO –2.68) and estimated glomerular filtration rate (eGFR; ABA 109, PBO 105 mL/min/1.73 m2) and a higher rate of sustained CR (sCR; ABA 48%, PBO 38%; hazard ratio estimate [95% CI] 1.41 [0.9942, 2.0068]) at 1 year. In this serum proteomic analysis, correlations between protein biomarkers, baseline (BL) disease characteristics and treatment outcomes were studied to better understand disease pathogenesis and treatment effects.
Methods: Serum samples from pts with SLE (ACR 1982 criteria)2 and biopsy-proven LN (122 ABA, 119 PBO) in ALLURE (NCT01714817) were analyzed for 122 protein biomarkers. Associations between biomarkers, categorical disease measurements and clinical responses were assessed by Wilcoxon-Mann-Whitney test, and between biomarkers and continuous disease activity measurements by Spearman’s rank correlation.
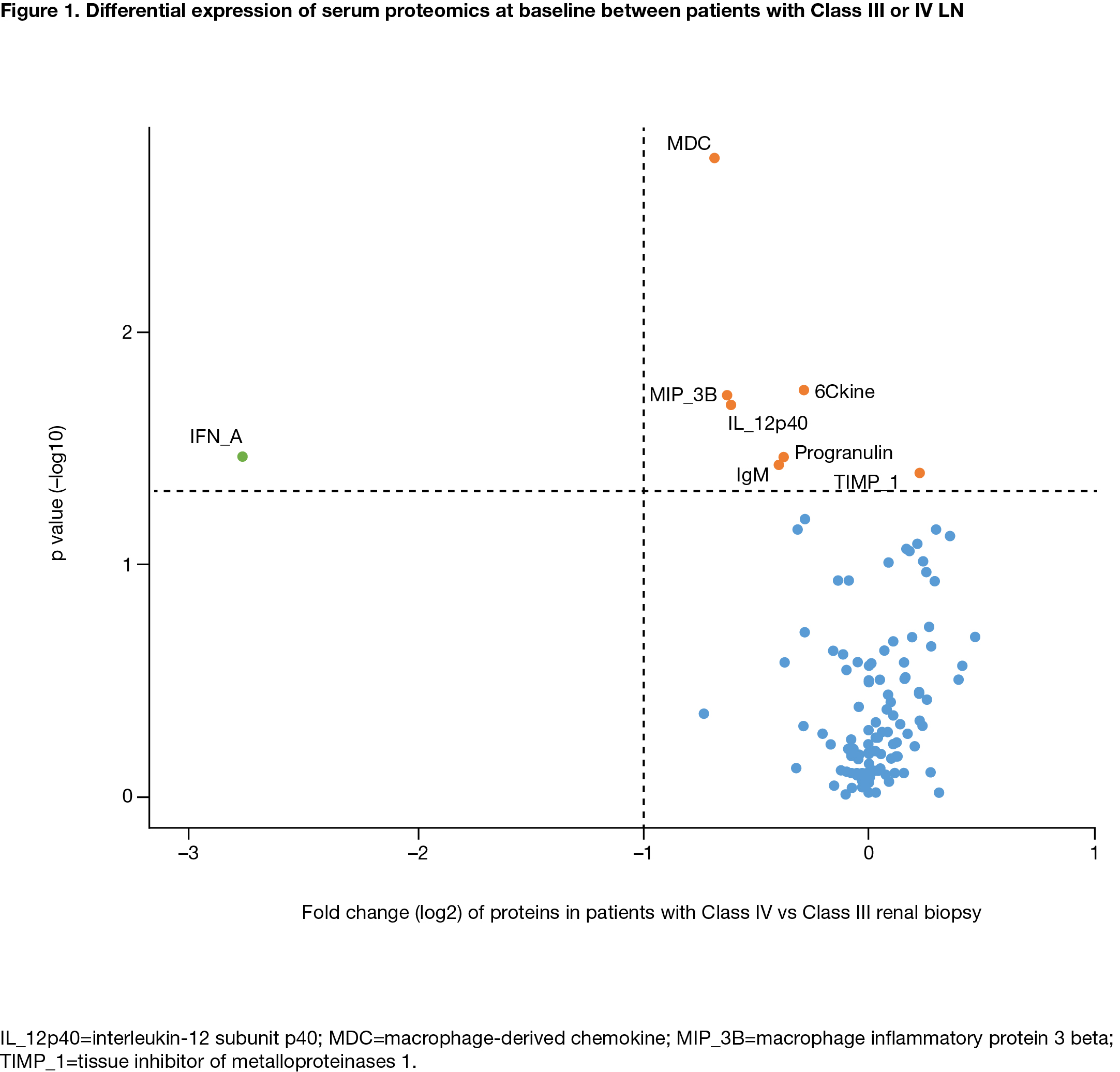
Results: Serum proteins at BL were comparable in both treatment groups. At BL, 17 serum proteins significantly correlated with UPCR, 33 with eGFR and 26 with BILAG score (adjusted p< 0.01); these included albumin, CD40, TNFR2, SCF and CD27. Several protein biomarkers were differentially expressed between pts with International Society of Nephrology and the Renal Pathology Society Class III and IV LN (Figure 1). Class IV LN was associated with lower levels of IFN-α, MDC, MIP-3B and IL12p14 than Class III LN, suggesting differential cytokine regulation. Some proteins (eg, TNFR1, SCF) correlated with changes from BL in eGFR and UPCR over time. Thirteen biomarkers, including MCP2, IGFBP2, AXL, SCF and MMP-2, correlated with early (Day 85) UPCR improvement in the ABA, but not PBO, group. Pts with lower BL CD40, B2M and MMP7 levels had better treatment outcomes for eGFR (achieving normal eGFR or >85% of the BL value) at Day 365 in both treatment groups. BL levels of myoglobin, CD27, APRIL, SCF, IGFBP7 and EGFR were associated with favorable eGFR outcomes in the ABA group, while TNFR1, Fib-1C, 6CKine, HCC4 and FGF23 were associated with favorable eGFR outcomes in the PBO group. Similar patterns were observed for categorical improvement of UPCR (< 0.5, or >50% decrease from BL) at Day 365 with different serum protein biomarkers. A panel of BL protein biomarkers was identified including CD40, CD27, TNFR2 and FAS that strongly correlated with the achievement of sCR by Day 365 in the ABA, but not PBO, group.
Conclusion: Pts with active LN had distinct serum proteomic profiles that correlated with features of LN and treatment outcomes. Specific associations with abatacept are consistent with its immunomodulatory mechanistic effects. Clinical trials should endeavor to apply precision medicine approaches to improve understanding of LN and treatment strategies.
References
- Furie R, et al. EULAR 2018. Oral OP0253.
- Tan EM, et al. Arthritis Rheum 1982;25:1271–1277.
Medical writing: Rachel Rankin (Caudex)
To cite this abstract in AMA style:
Wang S, Furie R, Dooley M, Wofsy D, Takeuchi T, Malvar A, Doria A, Romero-Díaz J, Chan T, Appel G, Jayne D, Hu S, Gao S, Maldonado M. Serum Proteomics from a Phase III, Randomized, Placebo-Controlled Study of Patients with Active Lupus Nephritis: Correlation with Baseline Disease Characteristics and Response to Therapy [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/serum-proteomics-from-a-phase-iii-randomized-placebo-controlled-study-of-patients-with-active-lupus-nephritis-correlation-with-baseline-disease-characteristics-and-response-to-therapy/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/serum-proteomics-from-a-phase-iii-randomized-placebo-controlled-study-of-patients-with-active-lupus-nephritis-correlation-with-baseline-disease-characteristics-and-response-to-therapy/