Session Information
Date: Monday, October 27, 2025
Title: (1221–1247) Pain in Rheumatic Disease Including Fibromyalgia Poster
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: For patients with lupus nephritis, active disease can be solely renal or include a range of extrarenal signs, such as arthritis or serositis, that can cause pain and decreased quality of life. However, the etiology of pain in the absence of extrarenal clinical activity remains in question, and no clinical trials have evaluated therapies for this potentially debilitating aspect of SLE. The Accelerating Medicines Partnership (AMP) provides an unprecedented opportunity to evaluate molecular mediators of disease in a well-phenotyped multi-ethnic multi-racial cohort of lupus patients enrolled at centers across the US. Accordingly, this study leveraged the AMP and AMP AIM lupus cohorts to evaluate serum proteins associated with patient reported pain specifically in patients without extrarenal clinical activity.
Methods: Pain was assessed using the PROMIS-29 pain interference questionnaire. To limit variability and focus on clinically meaningful outcomes, only patients without clinical extrarenal disease and PROMIS pain interference T scores < 50 (no reported pain interference) or > 55 (abnormal pain interference) were included. The AMP lupus nephritis cohort was used for the discovery analysis (n=28 without pain, n=50 with pain). The AMP AIM lupus cohort, most with renal disease but all chosen without any extrarenal clinical activity, was used for early validation (n=3 without pain, n=8 with pain). Serum proteins were measured using the Olink Explore HT platform. Assays with less than 5 values (AMP) and 1 value (AMP AIM) above limit of detection in either comparison group were excluded. Differential protein expression was evaluated using unadjusted linear models (limma) with candidate proteins considered at log2FC |1.5| and nominal p < 0.05. Pathways were assessed by gene set enrichment analysis (GSEA). An interferon signaling score was calculated by the Singscore method using the proteins in the Reactome “Interferon Signaling” pathway included in the Olink Assay.
Results: The clinical characteristics of the cohorts are described in Table 1. Differential expression analysis revealed 229 (219 up and 10 down) proteins significantly different between patients with vs. without pain and no extrarenal disease in the AMP cohort (Fig 1A). GSEA identified pathways related to innate immune signaling, including interferon, to be associated with pain (Fig 1B). In the validation AMP AIM cohort, there were 148 (101 up and 47 down) proteins significantly associated with pain in the absence of extrarenal activity (Fig 1C). Four proteins—CCL7, IL6, CXCL8, and HYDIN—were significantly elevated in the pain group across both cohorts (Fig 1D and Fig 2A), and all four have been previously linked to the expression of pain in the literature (Fig 2C). An interferon signaling score significantly associated with pain in AMP but not AMP AIM (Fig 2B).
Conclusion: These data suggest that circulating proteins may be key mediators of pain even in SLE patients without overt extrarenal clinical activity. Experimental evidence from mouse models supports the involvement of the identified candidates: CCL7 in neuropathic pain, IL6 in both neuropathic and nociceptive pain, CXCL8 in nociceptive pain, and HYDIN in nociceptive pain.
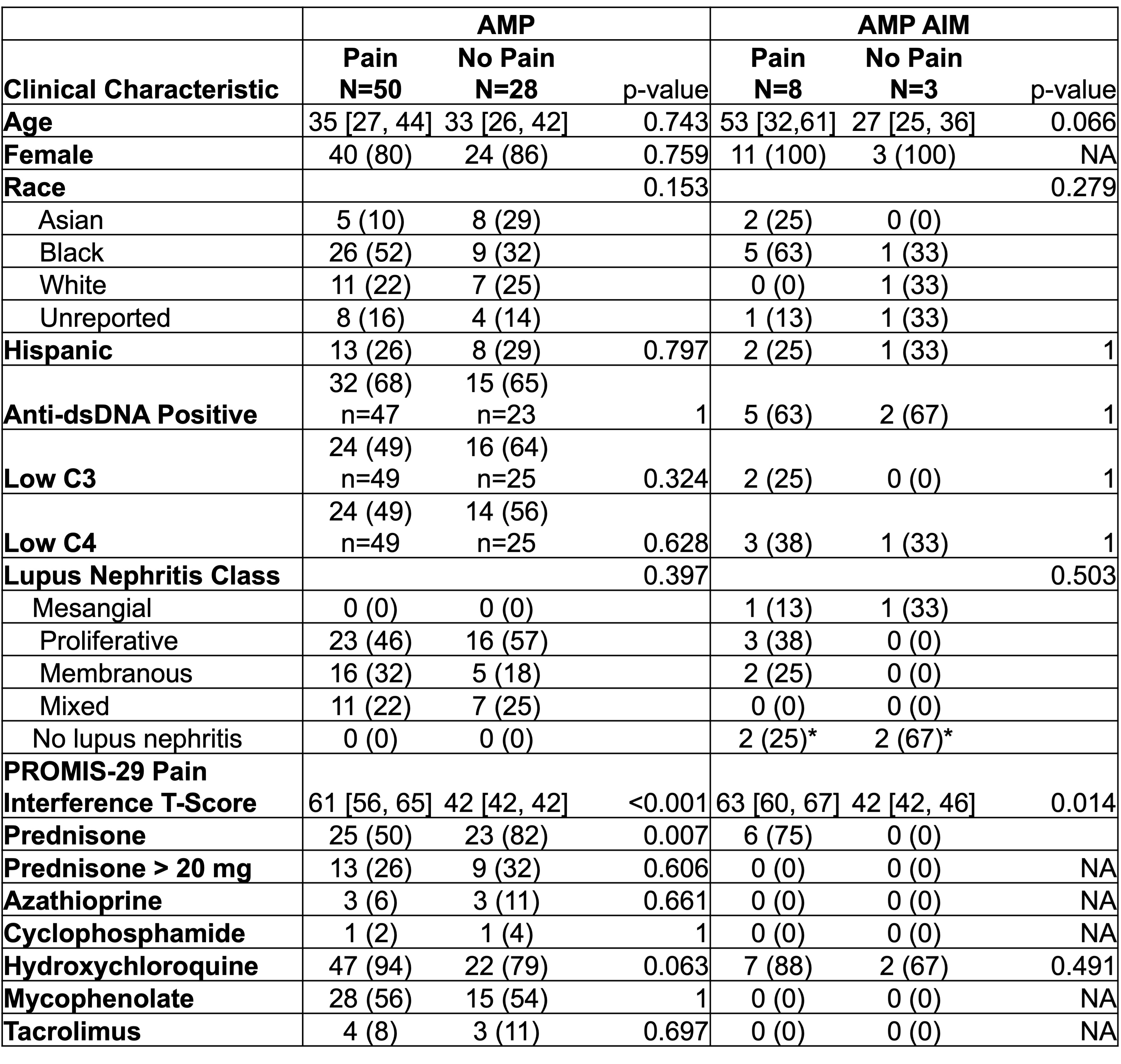 Table 1 Clinical characteristics of patients with and without reported pain in the AMP and AMP AIM lupus cohorts without extrarenal clinical activity. Data represented as median [IQR] or N (%). P-value calculated using chi-squared, Fisher’s exact, or Mann Whitney U test where appropriate. *1 biopsy with arteriosclerosis and 1 biopsy with an unknown pathology but not lupus nephritis.
Table 1 Clinical characteristics of patients with and without reported pain in the AMP and AMP AIM lupus cohorts without extrarenal clinical activity. Data represented as median [IQR] or N (%). P-value calculated using chi-squared, Fisher’s exact, or Mann Whitney U test where appropriate. *1 biopsy with arteriosclerosis and 1 biopsy with an unknown pathology but not lupus nephritis.
.jpg) Figure 1 (A) Volcano plot of AMP cohort comparison for Pain vs. No Pain (|logFC| > 1.5, p-value < 0.02). (B) GSEA results for the Reactome pathway database for AMP cohort comparison. (C) Volcano plot of AMP-AIM cohort comparison for Pain vs. No Pain (|logFC| > 1.5, p-value < 0.02). (D) Venn Diagram showing overlap between AMP and AMP AIM differential analysis results (|logFC| > 1.5, p-value < 0.05).
Figure 1 (A) Volcano plot of AMP cohort comparison for Pain vs. No Pain (|logFC| > 1.5, p-value < 0.02). (B) GSEA results for the Reactome pathway database for AMP cohort comparison. (C) Volcano plot of AMP-AIM cohort comparison for Pain vs. No Pain (|logFC| > 1.5, p-value < 0.02). (D) Venn Diagram showing overlap between AMP and AMP AIM differential analysis results (|logFC| > 1.5, p-value < 0.05).
.jpg) Figure 2 (A) Box plots of NPX (normalized protein expression) values for the in common differential proteins between AMP and AMP AIM cohorts when comparing Pain vs. No Pain. (B) Box plots of the Interferon Signaling Singscore for the AMP cohort. (C) Citations showing each of the four protein candidates has been previously associated with pain.
Figure 2 (A) Box plots of NPX (normalized protein expression) values for the in common differential proteins between AMP and AMP AIM cohorts when comparing Pain vs. No Pain. (B) Box plots of the Interferon Signaling Singscore for the AMP cohort. (C) Citations showing each of the four protein candidates has been previously associated with pain.
To cite this abstract in AMA style:
Keegan S, Carlucci P, Izmirly P, Carter E, Sanyal S, Cohen B, Shwetar J, Preisinger K, Zaminski D, Deonaraine K, Masson M, Fava A, James J, Lu R, DeJager W, Putterman C, Belmont M, Furie R, Dall'Era M, Kamen D, Kalunian K, Anolik J, Wofsy D, Barnas J, Hacohen N, Clancy R, Guthridge J, Rovin B, Petri M, Buyon J, Ruggles K. Serum Olink Proteomics Identifies Novel Mediators of Pain in Lupus Nephritis Patients Without Extrarenal Clinical Activity [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/serum-olink-proteomics-identifies-novel-mediators-of-pain-in-lupus-nephritis-patients-without-extrarenal-clinical-activity/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/serum-olink-proteomics-identifies-novel-mediators-of-pain-in-lupus-nephritis-patients-without-extrarenal-clinical-activity/
