Session Information
Date: Wednesday, November 13, 2019
Title: 6W023: Systemic Sclerosis & Related Disorder – Clinical III: Predictors of Outcome (2912–2917)
Session Type: ACR Abstract Session
Session Time: 11:00AM-12:30PM
Background/Purpose: Systemic sclerosis (SSc) is a highly heterogeneous disease orphan of effective disease modifying agents. The diffuse cutaneous clinical subset (dcSSc) is currently targeted in most clinical trials. Nevertheless, there is still high variability in clinical outcome within dcSSc, which is limiting effective clinical trial design and interpretation. Global Ranked Composite Score (GRCS) and Composite Response Index in SSc (CRISS) are two of the most recent attempts to capture overall response to treatment in dcSSc. Activation of interferon type 1 (IFN) pathway is associated with severe clinical manifestations in SSc. Microarray and proteomic studies have
indicated that the serum concentration of CCL2, CCL8, CCL19, CXCL9, CXCL10, and CXCL11 are the most relevant serum measures of IFN induced activation of PBMCs. Here we aimed to determine whether their serum concentration of the above chemokines combined in a IFN score could be used to stratify patients with dcSSc for severe clinical outcome at 12 months as measured by GRCS and CRISS.
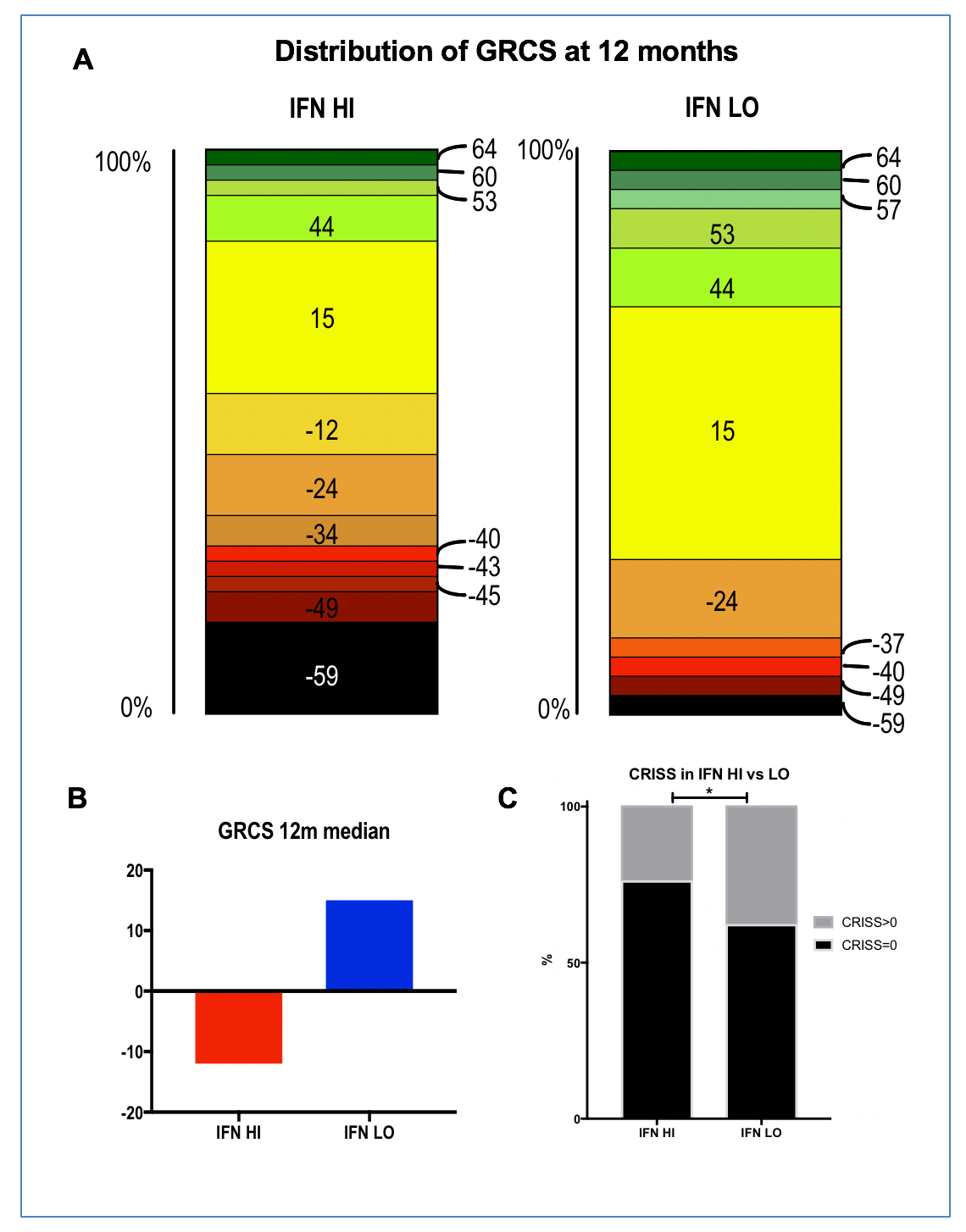
Methods: Serum concentration of CCL2, CCL8, CCL19, CXCL9, CXCL10, and CXCL11 was measured by Luminex xMAP technology (Myriad RBM) in 143 SSc patients and 35 healthy controls (HC). IFN score was calculated as the average of the natural logarithm of the above chemokines. GRCS were compared by Mann Whitney test and the effect size for the Mann Whitney test by Wilcoxon Paired ranked test, as described. Fisher exact test was used to analyse the proportion of patients with CRISS of 0% or >0%.
Results: All chemokines had a higher serum concentration in SSc vs HC (p< 0.0001 for all). Median IFN score was higher in SSc than HC (5.26 vs 4.70, p< 0.0001), but within SSc group, there was no difference associated with disease subset or duration. As with the dichotomization for IFN activity already described for RNA IFN Score, we defined IFN LO or HI patients as a score within or above mean + 2STDV of IFN Score of HCs. Sixty six 12-month outcome data of dcSSc patients were available for analysis. 37 were IFN HI and 29 IFN LO. GRCS ranged from -59 to 64. IFN HI patients had a worse outcome at 12 months with GRCS median score of -12 vs 15 in IFN LO (p=0.0271). Accordingly, GRCS favored IFN LO in 68.4% of 1073 (37*29) pairwise comparisons versus 31.6% of IFN HI (p=0.0001). 12 month CRISS was >0 in 24% of IFN HI vs 38% of IFN LO (P=0.0464).
Conclusion: Serum IFN Score predicts worse clinical outcome at 12 months in dcSSc. Stratification for IFN score could aid both in clinical trial design and clinical management. Moreover, here we show that GRCS and CRISS may be sufficiently sensitive to measure difference in composite outcome at 12 months in dcSSc in an observational setting.
To cite this abstract in AMA style:
Carriero A, Abignano G, Hutchinson M, Ballard K, Del Galdo F. Serum Interferon Score Predicts Clinical Outcome at 12 Months in Diffuse Cutaneous Systemic Sclerosis as Measured by Global Ranked Composite Score (GRCS) and Composite Response Index in SSc (CRISS) [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/serum-interferon-score-predicts-clinical-outcome-at-12-months-in-diffuse-cutaneous-systemic-sclerosis-as-measured-by-global-ranked-composite-score-grcs-and-composite-response-index-in-ssc-criss/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/serum-interferon-score-predicts-clinical-outcome-at-12-months-in-diffuse-cutaneous-systemic-sclerosis-as-measured-by-global-ranked-composite-score-grcs-and-composite-response-index-in-ssc-criss/