Session Information
Date: Monday, November 14, 2022
Title: Systemic Sclerosis and Related Disorders – Clinical Poster III
Session Type: Poster Session D
Session Time: 1:00PM-3:00PM
Background/Purpose: Type-I Interferon pathway activation has been associated with severity and progression of diffuse cutaneous SSc (dcSSc). The role of type-I Interferons (IFNs) in limited cutaneous systemic sclerosis (lcSSc) has not yet been explored. Here we aimed to determine the value of Type I IFN activation in stratifying for clinical outcome in lcSSc as assessed by a novel composite endpoint developed for the MINIMISE clinical trial of mycophenolate mofetil in lcSSc (EudraCT: 2019-004139-21)
Methods: Consecutive lcSSc patients from our observational cohort and age/sex- matched healthy controls (HC) were assessed for Type I IFN activation as measured by a serum chemokine interferon score calculated as the average of the logged serum concentration of CCL2, CCL8, CCL19, CXCL9, CXCL10, and CXCL11, as previously described. Patients were dichotomised in IFN Hi or Low according to scores over or within 1 Standard Deviation of the HC mean. The MINIMISE 3 year morbi-mortality endpoint included (i) New lung fibrosis on HRCT (FVC< 70%), (ii) Deterioration of established lung fibrosis, (iii) Significant progression of skin score, (iv) New major cardiac complication, (v) Scleroderma renal crisis, (vi) new diagnosis of pulmonary hypertension, (vii) Severe SSc related GI disease, (viii) Severe digital vasculopathy and (ix) Mortality. ANOVA, Pearson/Spearman correlation analysis, Fisher Exact Test (FET) and long-rank Mantel-Cox Chi Square (Mantel-Cox) were employed for statistical analysis, as appropriate.
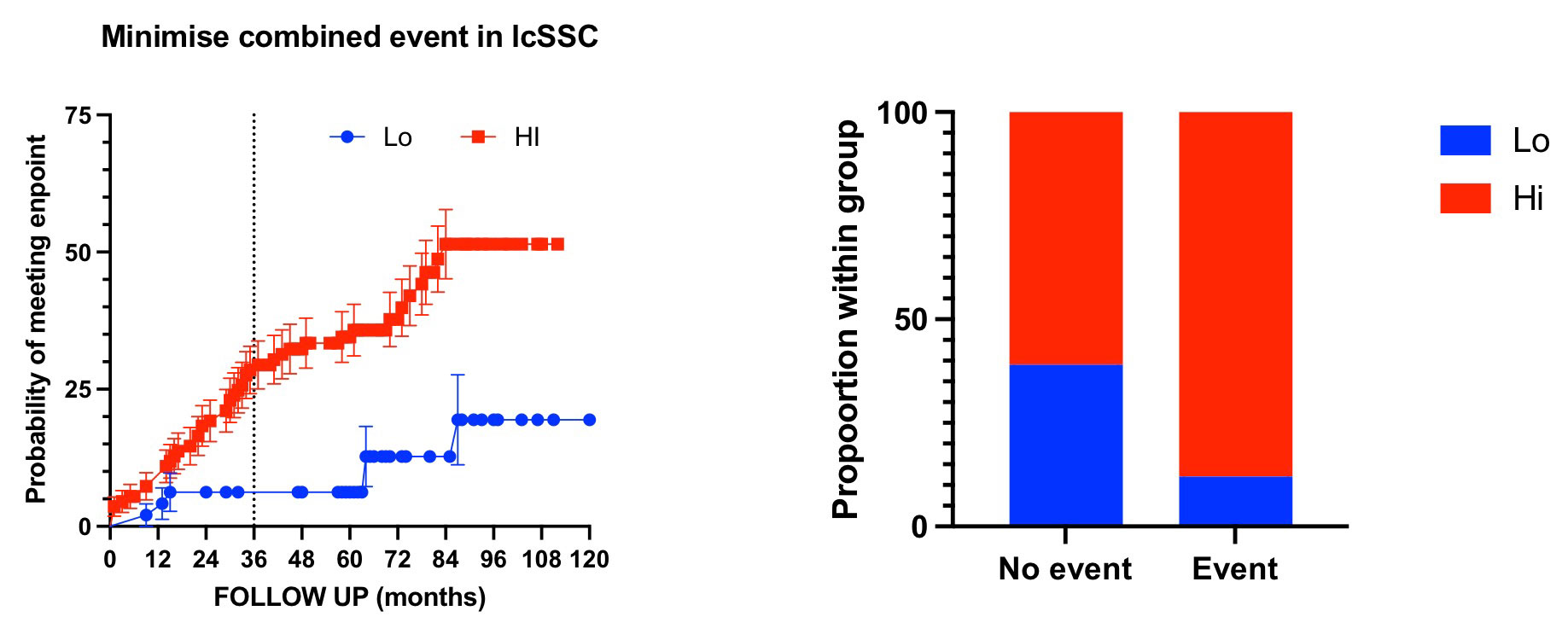
Results: 158 consecutive lcSSc patients enrolled in a single centre observational cohort were included in the analysis (149 F, mean age 65 ± 12years). Median (IQR) disease duration at time of IFN testing was 67 months (58,74), with mean (STDV) follow up of 75 (23) months. Mean (STDV) of IFN score in lcSSC was significantly higher than healthy controls [5.41(0.47) vs.4.86(0.32); P< 0.0001]. 111 (70%) patients were classified as IFN HI.
52/158 patients (33%) met the morbimortality event during the available follow up, of which 46 (88.5%) were IFN HI (FET = 0.0004). This gave patients with IFN HI a relative risk (95% C.I.) to meet the event of 3.32 (1.6 to 7.28). Restricting the analysis to the first 36 months after the test, 36 (22.8%) patients met the endpoint of which 33 (91.6%) where IFN Hi (FET P= 0.001). Therefore, the relative risk (95% C.I.) of patients with IFN Hi of meeting the MINIMISE endpoint in the first 3 years was 4.42 (1.6 to 13.21).
Median (IQR) time to event of IFN Hi patients was 29 months (14,60) vs 64 (26,85) of IFN low patients. Consistent with this analysis, survival (from combined endpoint) curve comparison showed a very significant difference (Mantel-Cox 11.62, p=0.0007)
Conclusion: Within the limitations of retrospective analysis of an observational cohort, our data suggest that 70% of patients with lcSSc show evidence of Type I IFN dysregulation. IFN Hi patients have worse outcome than IFN Lo as assessed by the MINIMISE combined endpoint analysis.
Independent validation in the context of the ongoing MINIMISE RCT will inform the use of IFN score as a stratification tool for lcSSc and to harmonise the scope for immune intervention and clinical management across both cutaneous subsets.
To cite this abstract in AMA style:
Karanth R, Abignano G, Kakkar V, Ross R, Denton C, Del Galdo F. Serum IFN Score Predicts Long Term Outcome in Limited Cutaneous Systemic Sclerosis [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/serum-ifn-score-predicts-long-term-outcome-in-limited-cutaneous-systemic-sclerosis/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/serum-ifn-score-predicts-long-term-outcome-in-limited-cutaneous-systemic-sclerosis/