Session Information
Session Type: Poster Session B
Session Time: 9:00AM-10:30AM
Background/Purpose: Although type 1 interferon (IFN) plays a pivotal role in the pathogenesis of systemic lupus erythematosus (SLE) and polymyositis/dermatomyositis (PM/DM) [1] as typically evidenced by elevated expression of type 1 IFN-stimulated genes (ISGs) in peripheral immune cells, contribution of serum factors to ISGs was not fully elucidated. We investigated the cytokine induction in multiple myeloid lineages by serum from patients with SLE or PM/DM using the whole blood stimulation system.
Methods:
Serum was collected from newly diagnosed, untreated, and clinically active adult patients with SLE (SLE group), anti-ARS antibody-positive PM/DM (ARS group), and anti-MDA5 antibody-positive DM (MDA5 group), and healthy controls (HCs) (n=10, respectively). Heparinized whole blood from healthy donors was incubated with control serum (10 v/v%), patient serum (10 v/v%), or IFNs (IFN-α, IFN-β, and IFN-γ) in the presence of protein transport inhibitor cocktail for six hours. Red blood cells were lysed and leucocytes were fixed at a single step. Intracellular staining was performed and expression of 11 cytokines in CD14+ monocytes, CD1c+ dendritic cells (DCs), and CD123+ DCs were analyzed using flow cytometry. For transcriptomic analyses, sorted CD14+ monocytes from healthy donor were incubated with control serum, patient serum, or IFN-β for four hours and bulk RNA-sequencing was performed (n=3). To evaluate significance of JAK-STAT pathway, whole blood from healthy donors was pre-incubated with baricitinib, a JAK1/2 inhibitor, stimulated with patient serum for six hours, and analyzed for cytokine expression as described above.
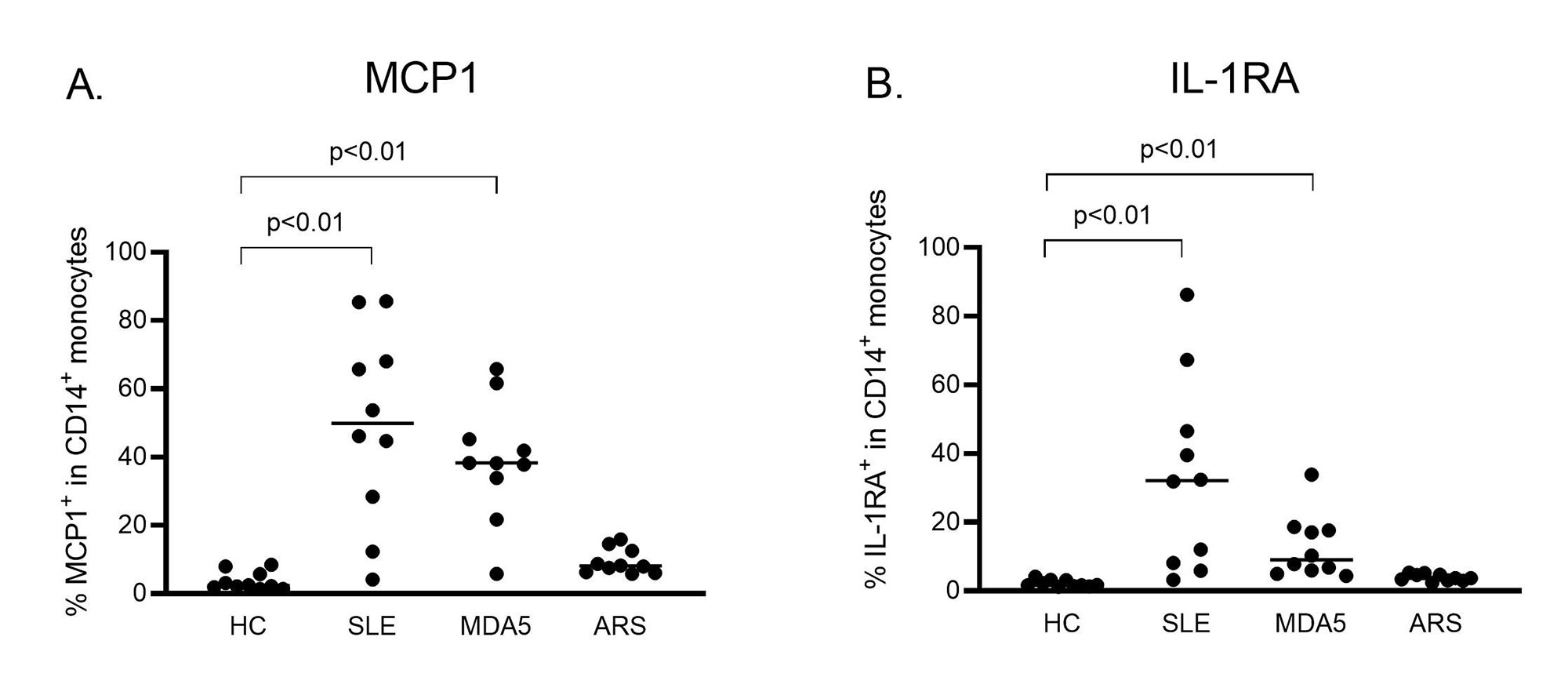
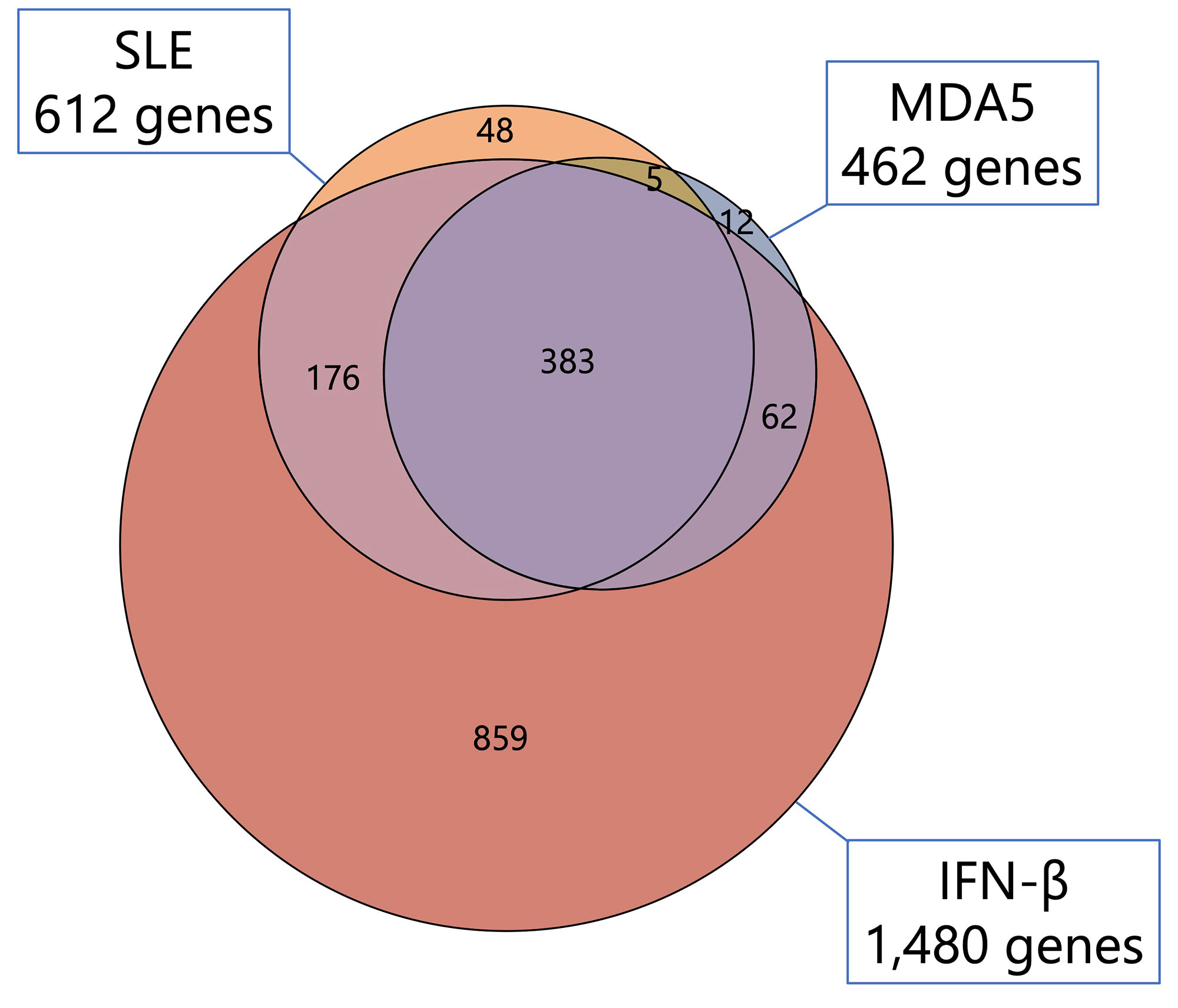
Results: Serum from SLE and MDA5 groups induced significantly higher monocyte chemoattractant protein-1 (MCP1) and interleukin-1 receptor antagonist (IL-1RA) expression in CD14+ monocytes compared to HCs (Figure 1). This monocyte cytokine signature was closely resembled that induced by IFN-β stimulation. Serum from ARS group did not induce any significant cytokine expression in CD14+ monocytes. No significant cytokine expression was observed in CD1c+ DCs and CD123+ DCs. Following stimulation with serum from SLE and MDA5 groups, 612 and 462 genes were elevated (fold change >2, p < 0.05 vs HCs), respectively. Among these elevated genes, 383 genes were commonly elevated between SLE group, MDA5 group, and IFN-β (Figure 2). Pathway analysis showed the highest number of gene elevation in IFN-αβ signaling pathway. Multiple ISGs as well as STAT1/2 were significantly elevated. In agreement with transcriptomic analysis, baricitinib significantly abrogated monocyte cytokine signature, MCP1 and IL-1RA expression, induced by serum from SLE and MDA5 groups (p < 0.05).
Conclusion: Serum from patients with clinically active SLE and anti-MDA5 antibody-positive DM induced shared monocyte cytokine signature through type 1 IFN pathway, suggesting contribution of serum factors to ISG signature in the peripheral CD14+monocytes. These ‘primed’ CD14+ monocytes by active serum could contribute to pathogenesis in these diseases.
[1] Antonios Psarras, et al. Rheumatology (Oxford). 2017;56(10):1662-1675
To cite this abstract in AMA style:
Nakamura S, okamoto Y, Katsumata Y, Harigai M. Serum from Patients with SLE and Anti-MDA5 Antibody-Positive Dermatomyositis Induce Shared Monocyte Cytokine Signature Through Type 1 Interferon Pathway [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/serum-from-patients-with-sle-and-anti-mda5-antibody-positive-dermatomyositis-induce-shared-monocyte-cytokine-signature-through-type-1-interferon-pathway/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/serum-from-patients-with-sle-and-anti-mda5-antibody-positive-dermatomyositis-induce-shared-monocyte-cytokine-signature-through-type-1-interferon-pathway/