Session Information
Session Type: Late-Breaking Abstract Poster Session
Session Time: 9:00AM-11:00AM
Background/Purpose: A non-drug biomarker study showed senescent cell (SnC) burden in OA synovial tissue to correlate with disease severity, inflammation, and knee pain (C. Yohn, Poster, this meeting). UBX0101 is a MDM2/p53 interaction inhibitor that can induce apoptosis of SnCs. Clearance of SnCs from joints by UBX0101 may create a pro-regenerative environment and lead to pain reduction. We assessed in a Phase 1 study the safety, pharmacokinetics (PK), and clinical outcomes of intra-articular (IA) UBX0101 treatment in patients (pts) with painful knee OA.
Methods: This was a Phase 1, double-blind, randomized, placebo-controlled, single, ascending dose (SAD) study in 48 pts randomized 3:1 to UBX0101 (dose range of 0.1 to 4.0 mg) or placebo IA. Key eligibility criteria included knee OA by ACR criteria, Kellgren-Lawrence grade (KLG) 1-4, and mean daily pain 4-9 on a Numeric Rating Scale (NRS, 0-10). Clinical outcomes through 12 weeks included WOMAC sub-scores for pain, function, and stiffness derived from the Knee Injury and Osteoarthritis Outcome Score (KOOS) Survey and NRS. PK modeling was used to pre-define low (0.1 to 0.4 mg) and high (1.0 to 4.0 mg) dose groups, predicted to achieve knee concentrations below and above pharmacological EC50 thresholds, respectively. Thirty additional subjects were randomized 2:1 to UBX0101 4.0 mg or placebo IA to assess the impact of UBX0101 on synovial fluid (SF) and plasma senescence-associated secretory phenotype (SASP) and OA disease biomarkers (BMKs). In this BMK sub-study, SF collected by arthrocentesis or lavage at Weeks 0 and 4 along with plasma were assayed using a custom multiplex panel. The SAD study design and sample size were chosen for evaluation of safety, while the BMK sub-study was sized to detect a meaningful change from baseline for BMKs.
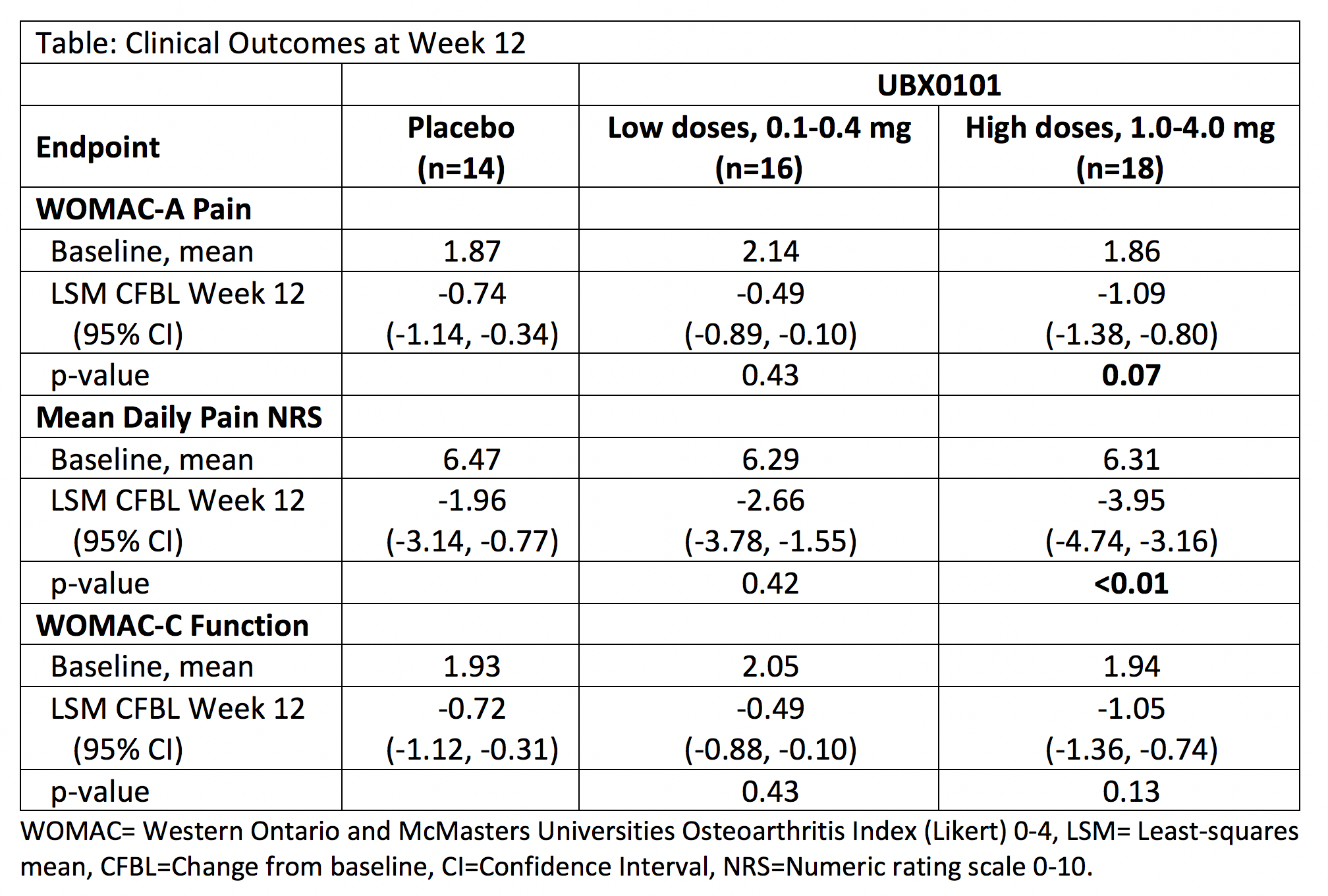
Results: The SAD study population was balanced regarding demographic and baseline OA characteristics; mean age was 62 years, 67% were female, 89.6% white. Single IA doses of UBX0101 up to 4 mg were well-tolerated. Most adverse events (AEs) were mild. No serious AEs occurred and no AEs led to discontinuation. The plasma PK of UBX0101 following single IA injection demonstrated minor interpatient variability at all dose groups and systemic concentrations were low and further minimized by a ~4 hour half-life. Improvements in pain and function were dose-dependent, clinically meaningful, and durable through 12 weeks for the three highest UBX0101 doses of 1.0, 2.0, and 4.0 mg (table). A greater proportion (50%) of pts in the high dose group achieved a 50% decrease in WOMAC pain sub-score at Week 12 compared to the placebo and low dose groups (36% and 25%, respectively). SF/lavage fluid analyses in the BMK sub-study revealed modulation of multiple SASP/OA markers such as tissue remodeling and inflammatory factors, after one dose of UBX0101 IA compared to placebo.
Conclusion: Single IA doses of UBX0101 up to 4.0 mg were well-tolerated by knee OA patients, and its PK was well-characterized. The activity of UBX0101 is supported by its effects on multiple BMKs, as well as the clinically meaningful and durable improvements in pain and function. UBX0101 as a senolytic agent may be an important future therapeutic option for pts with knee OA.
To cite this abstract in AMA style:
Hsu B, Visich J, Genovese M, Walter K, An M, Laberge R, Dananberg J. Safety, Tolerability, Pharmacokinetics, and Clinical Outcomes Following Single-Dose IA Administration of UBX0101, a Senolytic MDM2/p53 Interaction Inhibitor, in Patients with Knee OA [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/safety-tolerability-pharmacokinetics-and-clinical-outcomes-following-single-dose-ia-administration-of-ubx0101-a-senolytic-mdm2-p53-interaction-inhibitor-in-patients-with-knee-oa/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/safety-tolerability-pharmacokinetics-and-clinical-outcomes-following-single-dose-ia-administration-of-ubx0101-a-senolytic-mdm2-p53-interaction-inhibitor-in-patients-with-knee-oa/