Session Information
Date: Tuesday, October 23, 2018
Title: Metabolic and Crystal Arthropathies – Basic and Clinical Science Poster II
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
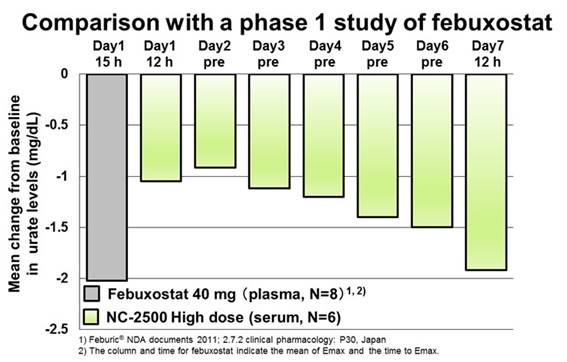
Background/Purpose: Gout flare due to rapid urate reduction after initiating urate-lowering therapy (ULT) is one of the major issues in the therapy. International guidelines recommend anti-inflammatory prophylaxis for gout flares during the initiation of ULT and colchicine is widely used. However, colchicine is potentially toxic and caution is advised. In addition, a recent study in Japan suggests stepwise dose increase of febuxostat from 10 to 20 then 40 mg/day is better than fixed-dose febuxostat 40 mg/day and is comparable with colchicine prophylaxis for the prevention of gout flares1, though the dosage of febuxostat in the US is 40 and 80 mg/day. NC-2500 is a novel potent XOR inhibitor and preclinical studies have shown that multiple doses increase the plasma concentration and enhance the urate-lowering effect of NC-2500 unlike febuxostat. This study aimed to assess the safety, tolerability, pharmacokinetics and pharmacodynamics (PD) profiles of NC-2500 in healthy volunteers.
Methods: A Phase 1, randomized, single-blind, placebo-controlled, single and multiple ascending dose study was conducted. Each cohort consisted of 8 subjects, with 6 receiving NC-2500 and 2 receiving placebo orally. A total of 5 cohorts were studied in the single-dose study (10 mg to 160 mg, fasted conditions) and 4 cohorts were studied in the multiple-dose study (10 mg to 80 mg, fed conditions). The levels of NC-2500 and uric acid in plasma/serum and urine were assayed at predetermined time points. Safety and tolerability were assessed by physical examination, vital signs, electrocardiography, clinical laboratory tests and adverse events (AEs).
Results: The maximum plasma concentration (Cmax) and the area under the plasma concentration-time curve (AUC) increased dose-dependently in the single and multiple dose studies. The time to reach the Cmax was approximately 2.0–3.0 hours for single dose and 3.0–5.0 hours for repeated doses. Following repeated dose, plasma concentrations of NC-2500 increased (AUC 1.4-fold and C max 1.5-fold at a maximum) in comparison with the first dose. NC-2500 was hardly excreted into the urine. The serum uric acid (sUA) levels were reduced after single and repeated administrations of NC-2500. Moreover, in repeated doses, the sUA levels decreased to a target range gradually over the 7 days. The incidence of AEs was similar between NC-2500 and placebo treatments and all AEs were mild in severity.
Conclusion: NC-2500 is expected to have potential to resolve the issues of current ULT by its unique PD profiles. As for safety, NC-2500 was considered safe and well-tolerated. Furthermore, NC-2500 was hardly excreted through the kidneys, which can be a favorable profile for patients with renal impairment, frequently observed in gout.
References: 1. Yamanaka et al. Ann Rheum Dis 2018.
To cite this abstract in AMA style:
Hirano M, Kobayashi S, Miyayama E, Ohta T, Yamamoto M, Yamakawa T. Safety, Pharmacokinetics and Pharmacodynamics of NC-2500, a Novel Xanthine Oxidoreductase Inhibitor, in Healthy Volunteers [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/safety-pharmacokinetics-and-pharmacodynamics-of-nc-2500-a-novel-xanthine-oxidoreductase-inhibitor-in-healthy-volunteers/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/safety-pharmacokinetics-and-pharmacodynamics-of-nc-2500-a-novel-xanthine-oxidoreductase-inhibitor-in-healthy-volunteers/