Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Sarilumab (SAR) is approved as monotherapy or in combination with conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) for treatment of patients (pts) with moderate-to-severely active rheumatoid arthritis (RA). In this post-marketing surveillance (PMS), real-world study in Japan, safety and effectiveness of SAR therapy for pts with RA were investigated.
Methods: Pts treated with SAR (200 or 150 mg once every 2 weeks) between June 2018 and November 2021 were enrolled and followed to November 2024. This analysis stratified pts by age (< 65 years [yrs], ≥65 to <75 yrs, ≥75 yrs) and evaluated treatment emergent adverse events (TEAEs) as priority surveillance items per the risk management plan of SAR in Japan. Serious infections, for which a causal relationship to treatment could not be excluded, were analyzed by stratification of pts by their lowest absolute neutrophil count (ANC) below/above the lower limit of normal (LLN; 1.96×103/µL) during the observation period for pts aged < 65 or ≥65 yrs. Effectiveness was assessed by 28-joint disease activity score with C-reactive protein (DAS28-CRP).
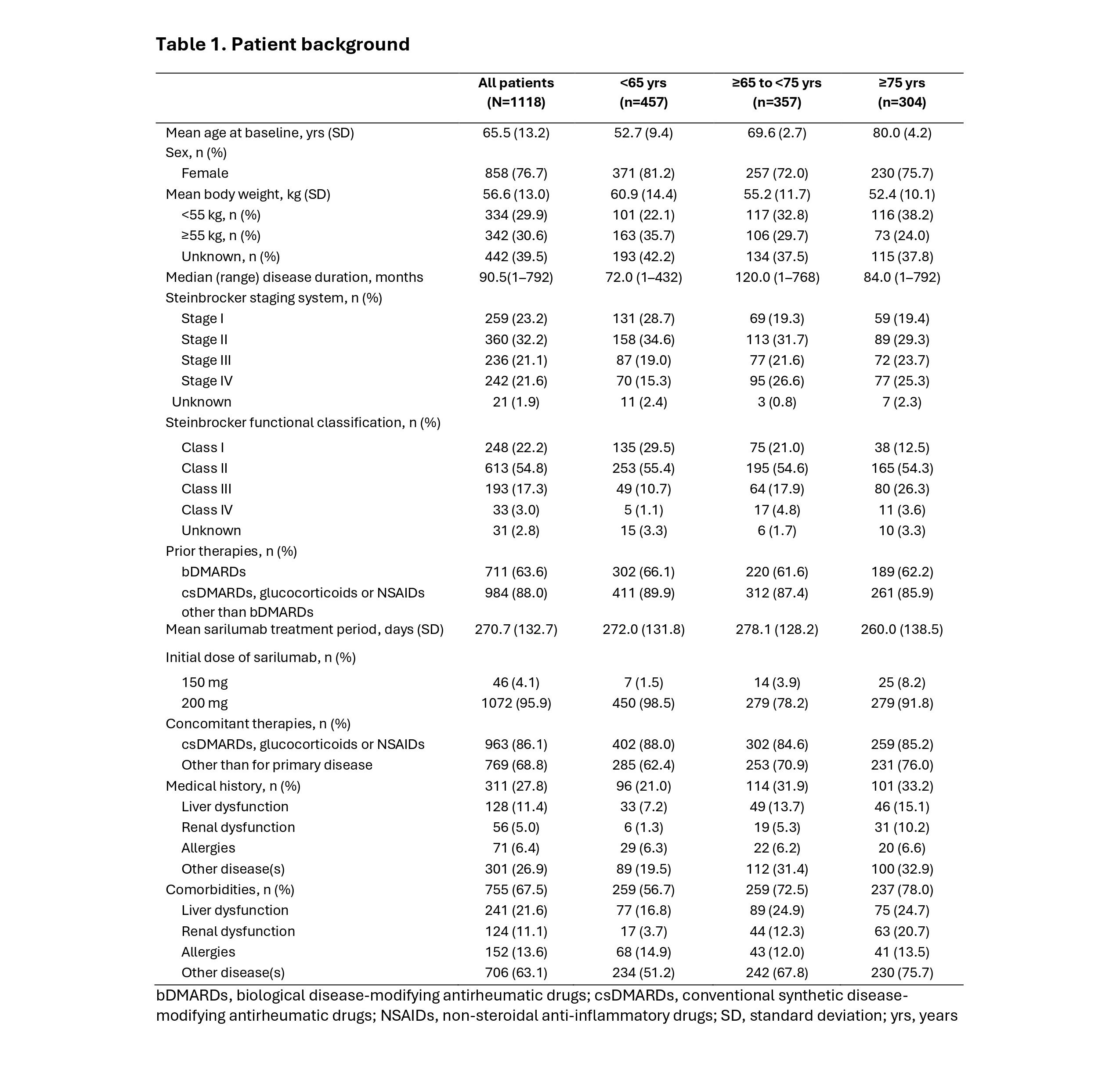
Results: Data were collected for 1121/1125 enrolled pts, with 62.1% (696/1121) who completed 1 yr of treatment (<65 yrs, 62.6% [288/460]; ≥65 to <75 yrs, 63.9% [228/357]; ≥75 yrs, 59.2% [180/304]). Major reasons for discontinuation varied by age for insufficient clinical effect (All, 15.3% [171/1121]; <65 yrs, 17.4% [80/460]; ≥65 to <75 yrs, 14.6% [52/357]; ≥75 yrs, 12.8% [39/304]) and TEAEs (All, 12.3% [138/1121]; <65 yrs, 9.3% [43/460]; ≥65 to <75 yrs, 12.3% [44/357]; ≥75 yrs, 16.8% [51/304]). The safety analysis included 1118 pts (female, 76.7% [858/1118]) (Table 1). Prior treatment with biologic DMARDs was observed in 63.6% (711/1118) of pts and was similar by age. Most pts received SAR 200 mg (95.9% [1072/1118]), with similar use of concomitant medications by age (All: 86.1% [963/1112] <65 yrs, 88.0% [402/457]; ≥65 to <75 yrs, 84.6% [302/357]; ≥75 yrs, 85.2% [259/304]). The incidence of priority surveillance items was 13.5% (151/1118) and tended to increase with age (< 65 yrs, 10.9% [50/457]; ≥65 to < 75 yrs, 15.4% [55/357]; ≥75 yrs, 15.1% [46/304]) (Table 2). Serious infections tended to increase slightly with age (< 65 yrs, 2.2% [10/457]; ≥65 to < 75 yrs, 4.2%, [15/357]; and ≥75 yrs, 5.6% [17/304]). Incidence of drug-related serious infections was similar when stratified by ANC ( >LLN, 2.9% [15/512] and < LLN, 1.7% [9/530]) (Table 3), with a similar trend by age (<65 yrs: >LLN, 2.0% [4/196] and < LLN, 1.3% [3/230]; ≥65 yrs: >LLN, 3.5% [11/316] and < LLN, 2.0% [6/300]). The incidence of treatment-related grade ≥3 neutropenia was also similar (< 65 yrs: 3.5% [16/457]; and ≥65 yrs: 3.9% [26/661]). In 1037 pts eligible for effectiveness analysis, DAS28-CRP mean scores improved from 4.2 at baseline to 2.7 (low disease activity) after 1 month and to 2.3 (remission) at the final evaluation.
Conclusion: These results show that SAR treatment was well tolerated by pts in Japan with RA regardless of age, and no new safety concerns were identified. Additionally, no link was established between occurrence of neutropenia during SAR treatment and the development of serious infections.
To cite this abstract in AMA style:
Kameda H, Tasaka S, Takahashi T, Soeda N, Suzuki K, Tanaka Y. Safety of sarilumab in more than 1000 patients with rheumatoid arthritis in Japan by age group: a post-marketing surveillance study [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/safety-of-sarilumab-in-more-than-1000-patients-with-rheumatoid-arthritis-in-japan-by-age-group-a-post-marketing-surveillance-study/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/safety-of-sarilumab-in-more-than-1000-patients-with-rheumatoid-arthritis-in-japan-by-age-group-a-post-marketing-surveillance-study/

.jpg)
.jpg)