Session Information
Session Type: Abstract Session
Session Time: 3:00PM-4:30PM
Background/Purpose:
Systemic autoimmune diseases are based on an aberrant activation of B cells. Autologous CD19 chimeric antigen receptor (CAR) T cells allow deep depletion of B cells in humans and represent a new possibility to treat autoimmune disease. Previous observations have suggested that a single infusion of CD19-CAR-T cells is not only well tolerated in patients with SLE and other autoimmune diseases but also induces sustained drug-free remission. However, safety and efficacy of CD19-CAR-T cell therapy in autoimmune disease has to be demonstrated in controlled clinical studies.
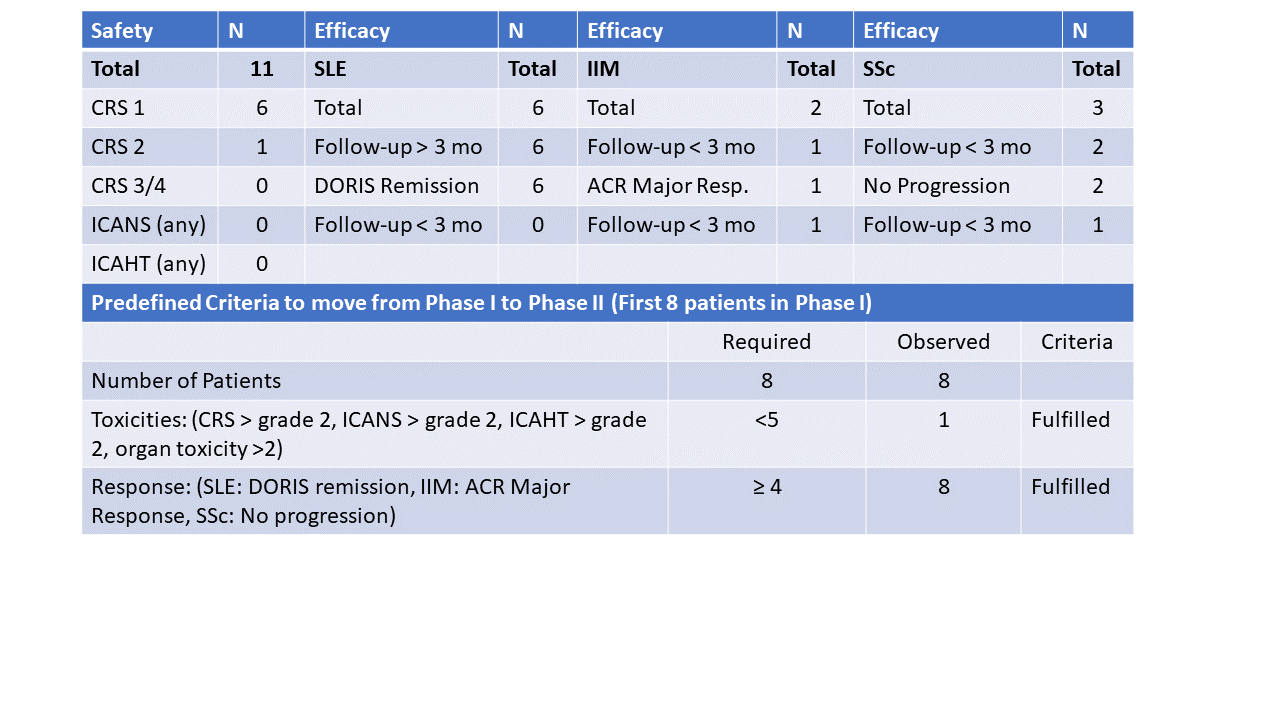
Methods: CASTLE (CAR-T cells in systemic B cell-mediated autoimmune disease) is a phase I/II basket study that assesses the safety (1° endpoint) and preliminary efficacy (2° endpoint) of CD19-CAR-T therapy in progressive, refractory systemic lupus erythematosus (SLE), idiopathic inflammatory myositis (IIM) and systemic sclerosis (SSc). CASTLE consists of a first phase with 8 patients, followed by a second phase with 16 patients, if toxicity is acceptable (< 5 toxicity events such as cytokine-release syndrome (CRS) > grade 2, immune effector cell-associated neurotoxicity syndrome (ICANS) > grade 2, grade III/IV neutropenia/leukocytopenia >28 days and organ toxicity >2) and efficacy is good (≥ 4 responders). Standard cyclophosphamide/fludarabine conditioning therapy was followed by a single infusion of an advanced therapy medicinal product (MB-CART19.1) containing 1×106 CD19-CAR-T cells/kg body weight. Safety was assessed by recording CRS, ICANS, myelotoxicity and organ toxicity during the first 28 days. Preliminary efficacy was assessed by assessing B cell depletion, CAR-T cell expansion and clinical responses.
Results: This interim analysis comprises 11 patients (6 SLE, 3 SSc, 2 IIM) from 1st phase and early 2nd phase. Median age was 38 years (20-81), median disease duration 3 years (0.5-13.5), median follow up time 6 months (1-9). From all patients, toxicity data, B cell depletion and CAR-T cell expansion data were available, while clinical efficacy data were available from 9/11 patients with sufficiently long follow-up (3 months). No higher-grade CRS (grade 3 or 4) was observed (grade 0: N=4; grade 1: N=6; grade 2: N=1). No ICANS, no myelotoxicity occurred. CAR-T cells expanded in all patients. Among 9 patients (6 SLE, 2 SSc, 1 IIM) that had sufficiently long follow-up (≥ 3 months), all 6 SLE patients achieved DORIS remission, the one IIM patient achieved ACR Moderate/Major response and both SSc patients showed no worsening of lung function (SSc) and improvement in skin scores. Furthermore, all patients could successfully stop glucocorticoids and immunosuppressive drugs after CAR-T cell infusion. Overall, the predefined endpoints required to fulfill the first phase (N=8 patients) of CASTLE (< 5 toxicity events; ≥ 4 responders) were met and the 2nd phase of CASTLE started, 3 additional patients were included into the 2nd phase of CASTLE.
Conclusion:
These data underline the safety of CD19-CAR-T therapy in the treatment of autoimmune disease. No higher-grade CRS or ICANS and no myelotoxicity was observed. Also, the preliminary efficacy data met the expectations in SLE, IIM and SSc.
References: Mueller F et al. N Engl J Med 2024; 390:687-700
To cite this abstract in AMA style:
Schett G, Müller F, Hagen M, Wirsching A, Bohr D, Bergmann C, Tur C, Völkl S, Aigner M, Kretschmann S, Spoerl S, Kharboutli S, Vasova I, Aletaha D, Kiener H, Natalello G, Locatelli F, D´Agostino M, Bozec A, Grieshaber-Bouyer R, MAckensen A. Safety and Preliminary Efficacy of CD19 CAR-T Cell Treatment in Rheumatic Disease – Data from the First Part of the Phase I/II CASTLE Basket Study [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/safety-and-preliminary-efficacy-of-cd19-car-t-cell-treatment-in-rheumatic-disease-data-from-the-first-part-of-the-phase-i-ii-castle-basket-study/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/safety-and-preliminary-efficacy-of-cd19-car-t-cell-treatment-in-rheumatic-disease-data-from-the-first-part-of-the-phase-i-ii-castle-basket-study/