Session Information
Session Type: Abstract Session
Session Time: 3:00PM-4:30PM
Background/Purpose: Treating autoimmune diseases like Systemic Lupus erythematosus (SLE), Idiopathic Inflammatory Myositis (IIM) or Systemic Sclerosis (SSc) is challenging and often requires life-long immunosuppressive therapy. In some patients, autoimmune disease progresses fast and leads to severe organ damage. Recent experience suggests that CD19-CAR T-cell represents a potentially transformative new therapy approach in autoimmune diseases, yet patient numbers and length of follow-up are limited.
Methods: Patients with treatment-refractory, progressive systemic SLE, IIM and SSc were treated with autologous CD19 CAR-T cell therapy in named patient use program (N = 19) or in phase I CASTLE trial (N = 11) (EudraCT-Nr:2022-001366-35) after fludarabine and cyclophosphamide lymphodepletion. All immunosuppressive therapies were stopped at least two weeks prior to leukapheresis. Prevalence and severity of cytokine-release syndrome (CRS), immune effector cell-associated neurotoxicity syndrome and immune effector cell–associated hematotoxicity syndrome (ICAHTS) as well as the efficacy of CAR T-cell treatment was documented.
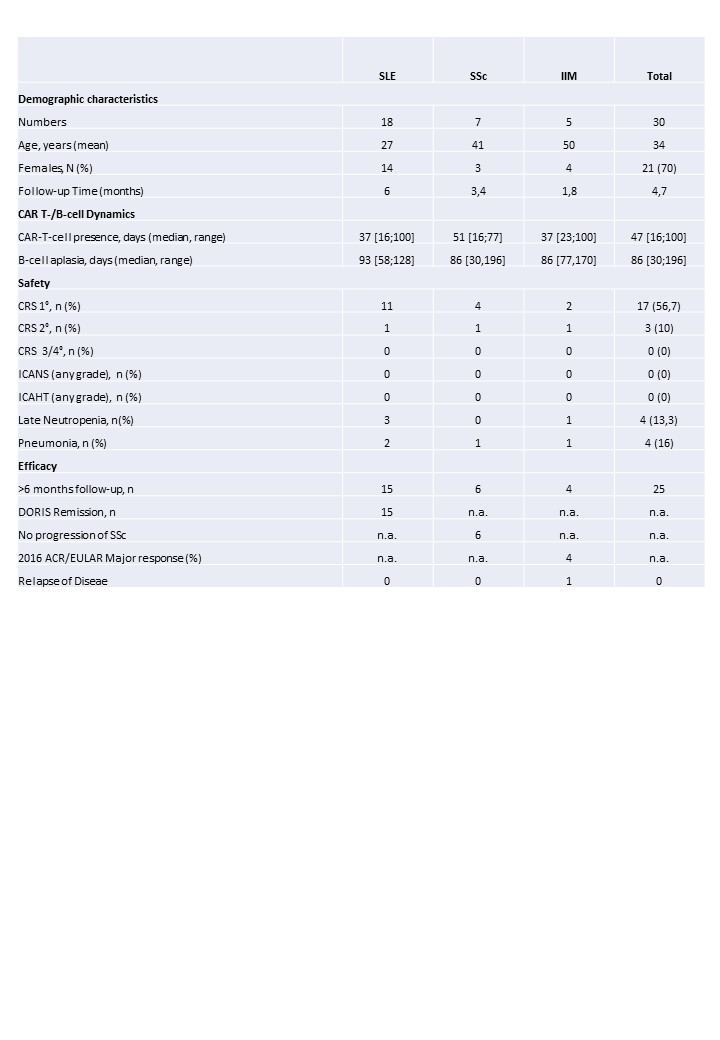
Results: 30 patients (18 SLE, 7 SSc, 5 IIM), among them two pediatric SLE patients, were treated with CD19-CAR T-cells between 2021 and 2024. All patients showed uncontrolled severe disease despite exposure to multiple drugs (Figure 1). Median follow-up was 12,5 months [range 1;39]. In the 26 patients with complete CAR T-cell involution (< 1 cell/microliter), mean time of CAR T-cell presence was 47 days [range 16;100]. In the 23 patients with B-cell reconstitution, mean time of B-cell aplasia was 86 days [range: 30;196]. Regarding safety, only mild CRS (56,7% grade 1; 10% grade 2) and no ICANS (previously reported one case of ICANS grade 1 not confirmed) and no ICAHT were observed. Infections were mild, mostly upper respiratory tract infections while 4 patients had an uncomplicated pneumonia after CD19-CAR T-cell treatment. Late neutropenia was observed in 4 patients with a median of 71 days [29-120] after CD19-CAR T-cell treatment and fast reversibility upon G-CSF treatment. In patients with at least 6-month follow-up (N=25), all SLE patients achieved DORIS remission, all myositis patients achieved 2016 ACR/EULAR major response and none of the SSc patients showed progression of interstitial lung disease (Table 1). Only one relapse with mild myositis in an IIM patient was observed after being 15 months in drug-free remission.
Conclusion: Dynamics of CAR T-cell expansion and involution and B-cell ablation and recurrence is consistent among the patients. Tolerability of CAR T-cell therapy in autoimmune disease has been high with no higher-grade toxicities. Efficacy analysis showed long-standing drug-free remission (SLE and IIM) or no progression (SSc) with only very little recurrence of disease even years after the intervention.
References: 1. Schett G, Mackensen A, Mougiakakos D. CAR T-cell therapy in autoimmune diseases. Lancet. 2023 Nov 25;402(10416):2034-44. 2. Müller F, Taubmann J, Bucci L, et al. CD19 CAR T-Cell Therapy in Autoimmune Disease – A Case Series with Follow-up. N Engl J Med. 2024;390(8):687-700.
To cite this abstract in AMA style:
Hagen M, Müller F, Wirsching A, Tur C, Krickau T, Metzler M, Bucci L, Bergmann C, Auth J, Taubmann J, Garantziotis P, Boeltz S, Kharboutli S, Spoerl S, Völkl S, Aigner M, Kretschmann S, Vasova I, MAckensen A, Grieshaber-Bouyer R, Schett G. Safety and Long-term Efficacy of CD19-CAR T-cell Therapy in 30 Patients with Autoimmune Disease [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/safety-and-long-term-efficacy-of-cd19-car-t-cell-therapy-in-30-patients-with-autoimmune-disease/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/safety-and-long-term-efficacy-of-cd19-car-t-cell-therapy-in-30-patients-with-autoimmune-disease/