Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Immunosuppressed Sjögren’s disease (SjD) patients are at increased risk of herpes zoster (HZ). Despite this vulnerability, data on safety and immunogenicity of the recombinant HZ vaccine (RZV, Shingrix®) in these patients remain limited. Concerns about disease flares following vaccine, as highlighted in studies of COVID-19 vaccination, further underscore the need for target evaluation. Purpose: To evaluate the safety and immunogenicity of RZV in immunosuppressed SjD.
Methods: In this randomized, double-blind, placebo-controlled trial, 67 adults immunosuppressed SjD patients (excluding rituximab) were enrolled, alongside 201 non-immunosuppressed control individuals (CG). SjD patients were randomized to receive vaccine (P1) or placebo (P2) at day 0 (D0) and D42, with placebo recipients vaccinated after unblinding (D84 and D126). CG were immunized at D0 and D42. Disease activity [ESSDAI (European League Against Rheumatism-EULAR Sjögren’s Syndrome Disease Activity Index)] and adverse events (AEs) were monitored at all visits. Humoral immunogenicity was assessed by serum anti-gE antibody concentrations using a GSK ELISA. The humoral response to VZR was defined as anti-gE concentration ≥4-fold the pre-vaccination concentration. Geometric Mean Titer (GMT) and Factor Increase (FI-GMT) were calculated.
Results: Vaccine (P1) and placebo (P2) groups were comparable in terms of age (p=0.5), sex (p=1.0), prednisone use (p=0.3) and dosage (0.6), as well as treatment with hydroxychloroquine (p=0.7), methotrexate (p=0.5), leflunomide (p=0.4), azathioprine (p=0.5), and mycophenolate mofetil (p=0.8). Baseline disease activity, assessed by ESSDAI, was similar between P1 and P2 [2.0 (0.0-2.0) vs. 1.0 (0.0-2.5), p=0.7], and most patients presented low disease activity (ESSDAI < 5) in both groups (87.1% vs. 88.9%, p=1.0), respectively. No significant changes in ESSDAI were observed six weeks after the 1st (p=0.5) or 2nd (p=0.2) RZV dose. Flare rates, defined as increase ≥3 points in ESSDAI and/or increased glucocorticoid/immunosuppressant therapy, were also comparable between groups throughout the study (p >0.05). Regarding AEs, after the 1st RZV dose, local reactions were less frequent among SjD than in CG (70.1% vs. 84.6%, p=0.009), as were systemic reactions (50.7% vs. 62.2%, p=0.1). After the 2nd RZV dose, the rates were comparable between groups (p >0.05). No serious AEs were observed. Analysis of immunogenicity comparing SjD and age [55.0 (48.3-63.8) vs. 55.0 (52.0-64.0) years, p=0.1] and sex (female: 95.5% vs. 90.5%, p=0.3, respectively) balanced CG revealed a reduced humoral response in SjD patients (90.6% vs. 99.5%, p=0.0009). Furthermore, GMT and FI-GMT were significantly lower in SjD patients compared to controls (Table).
Conclusion: RZV demonstrated an excellent safety profile in immunosuppressed SjD patients, without triggering significant disease flares. Although the vaccine induced a high rate of humoral response, the magnitude of the response was significantly lower compared to healthy controls, which may have implications for long-term protection. (ClinicalTrials NCT05879419).
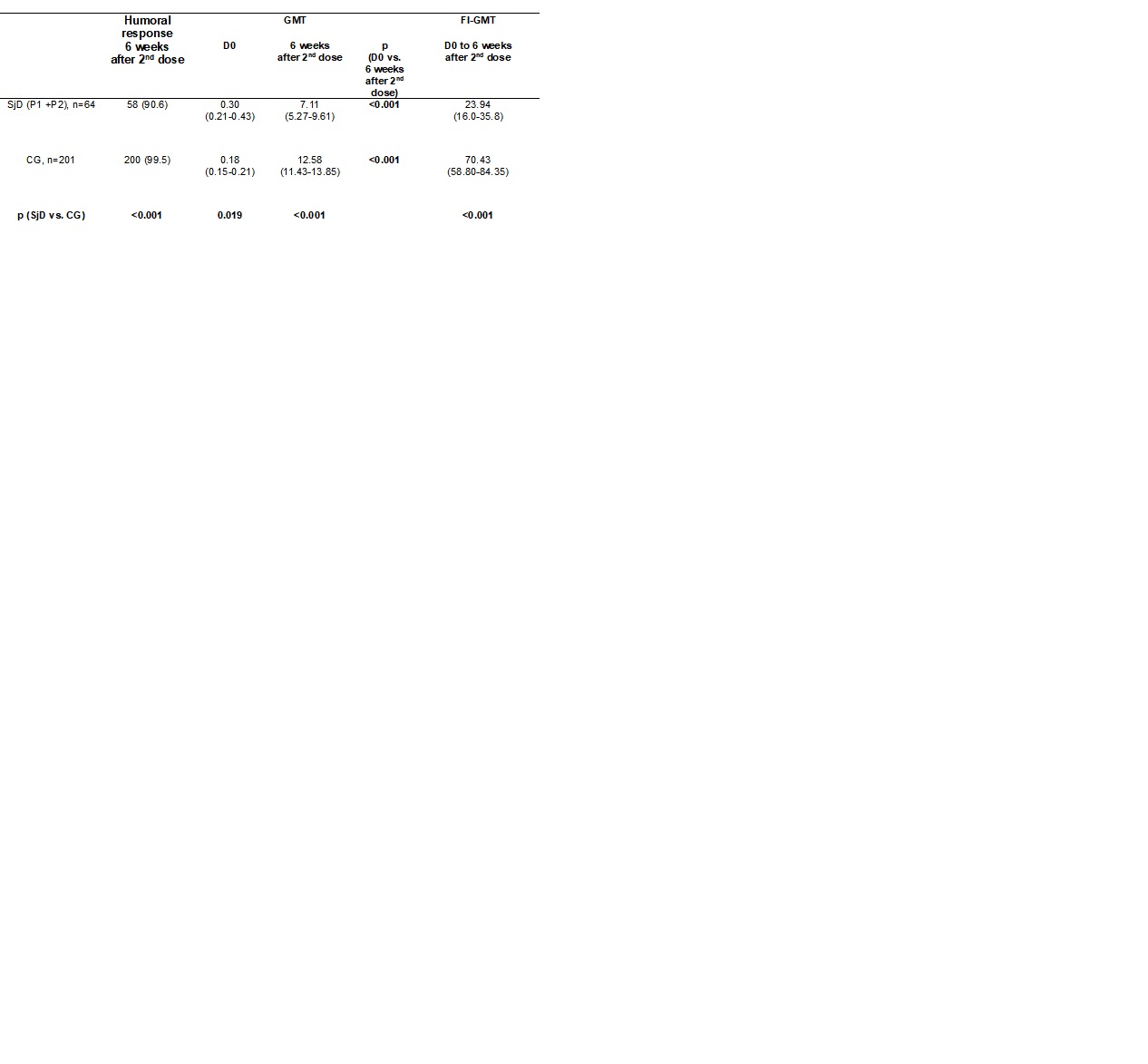 Table. Humoral response rates 6 weeks after the 2nd RZV dose and anti-gE IgG titers before (D0) and after two doses in patients with SjD and CG
Table. Humoral response rates 6 weeks after the 2nd RZV dose and anti-gE IgG titers before (D0) and after two doses in patients with SjD and CG
CG – control group, SjD – Sjögren’s disease. RZV – recombinant herpes zoster vaccine, GMT – geometric mean titers (mIU/mL).
Frequencies of humoral response are presented as number (%) and they were compared using two-sided chi-square test between SjD and CG at 6 weeks after the 2nd RZV dose.
Anti-gE IgG titers and factor increase (FI) in GMT are expressed as geometric means with 95% confidence interval (95%CI).
Data regarding anti-gE titers were analyzed using ANOVA with repeated measures and 2 factors [2 groups (SjD vs. CG), at 2 time points (D0 and 6 weeks after the 2nd RZV dose)], followed by Bonferroni’s multiple comparisons at neperian logarithm (ln)-transformed data. GMT and FI-GMT were compared using Mann-Whitney test for intergroup comparisons in ln-transformed data at pre-specified time points (D0 and 6 weeks after de 2nd dose). All analyses were two-sided.
P1 – SjD patients who received the RZV on the day of randomization (D0) and after 6 weeks (D42).
P2 – SjD patients who initially received placebo and were vaccinated 12 weeks after randomization, on D84 and after 6 weeks (D126).
To cite this abstract in AMA style:
Pasoto S, Ribeiro T, Aikawa N, Medeiros-Ribeiro A, Borges B, Franco A, Silva H, Bonfa E, Silva C. Safety and Humoral Response to Recombinant Herpes Zoster Vaccine in immunosuppressed Sjögren’s Disease Patients: Results From a Double-Blinded Placebo-Controlled Study [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/safety-and-humoral-response-to-recombinant-herpes-zoster-vaccine-in-immunosuppressed-sjogrens-disease-patients-results-from-a-double-blinded-placebo-controlled-study/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/safety-and-humoral-response-to-recombinant-herpes-zoster-vaccine-in-immunosuppressed-sjogrens-disease-patients-results-from-a-double-blinded-placebo-controlled-study/
