Session Information
Date: Tuesday, November 14, 2023
Title: (2387–2424) Vasculitis – Non-ANCA-Associated & Related Disorders Poster III
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: We recently found extracellular mitochondrial-derived N-formyl methionine in patients with giant cell arteritis (GCA. Extracellular mitochondria can be extruded by several mechanisms, including platelet activation, and are highly immunogenic, leading to formation of anti-mitochondrial antibodies (AMAs), such as anti-cardiolipin antibodies that have also been observed in GCA. The purpose of this study was to determine the presence of additional AMAs, including anti-mitofusin 1 (MFN1) antibodies, in patients with GCA. We also sought to determine whether extracellular mitochondria are capable of mediating platelet activation in patients with GCA.
Methods: Anti-MFN1 IgG levels were measured using an in-house ELISA in the plasma from patients with GCA (n=65 in remission, n=14 with active disease), and 30 healthy controls (HC). Ultrapure mitochondria, isolated from HepG2 cells, were incubated with plasma from 65 patients with GCA in remission, and 16 HC, and assessed for IgA and IgG binding to the mitochondrial outer membrane using flow cytometry. Mitochondria were also opsonized with patient (n=58) or healthy control (n=10) plasma (6%), and subsequently incubated with platelets to determine the capacity of plasma factors, including AMAs, to promote mitochondrial-mediated platelet activation.
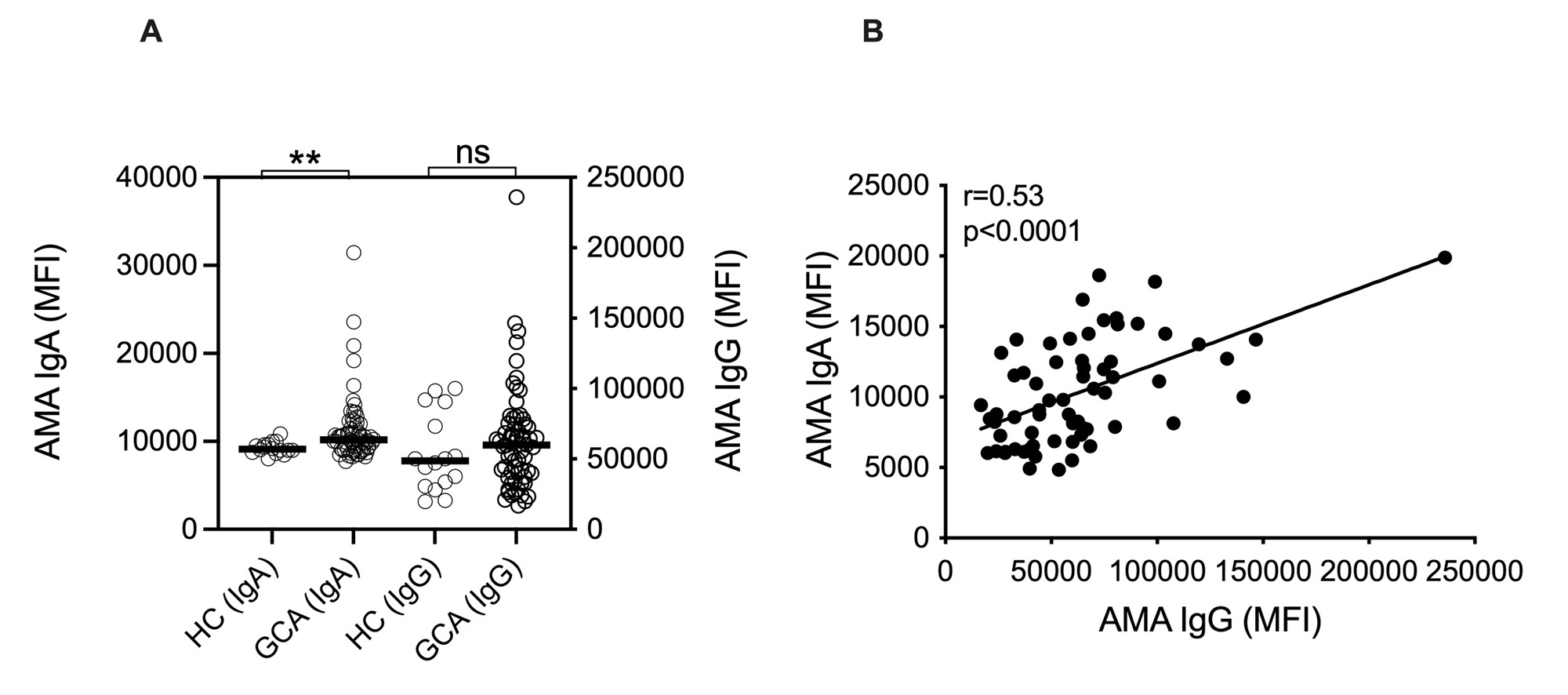
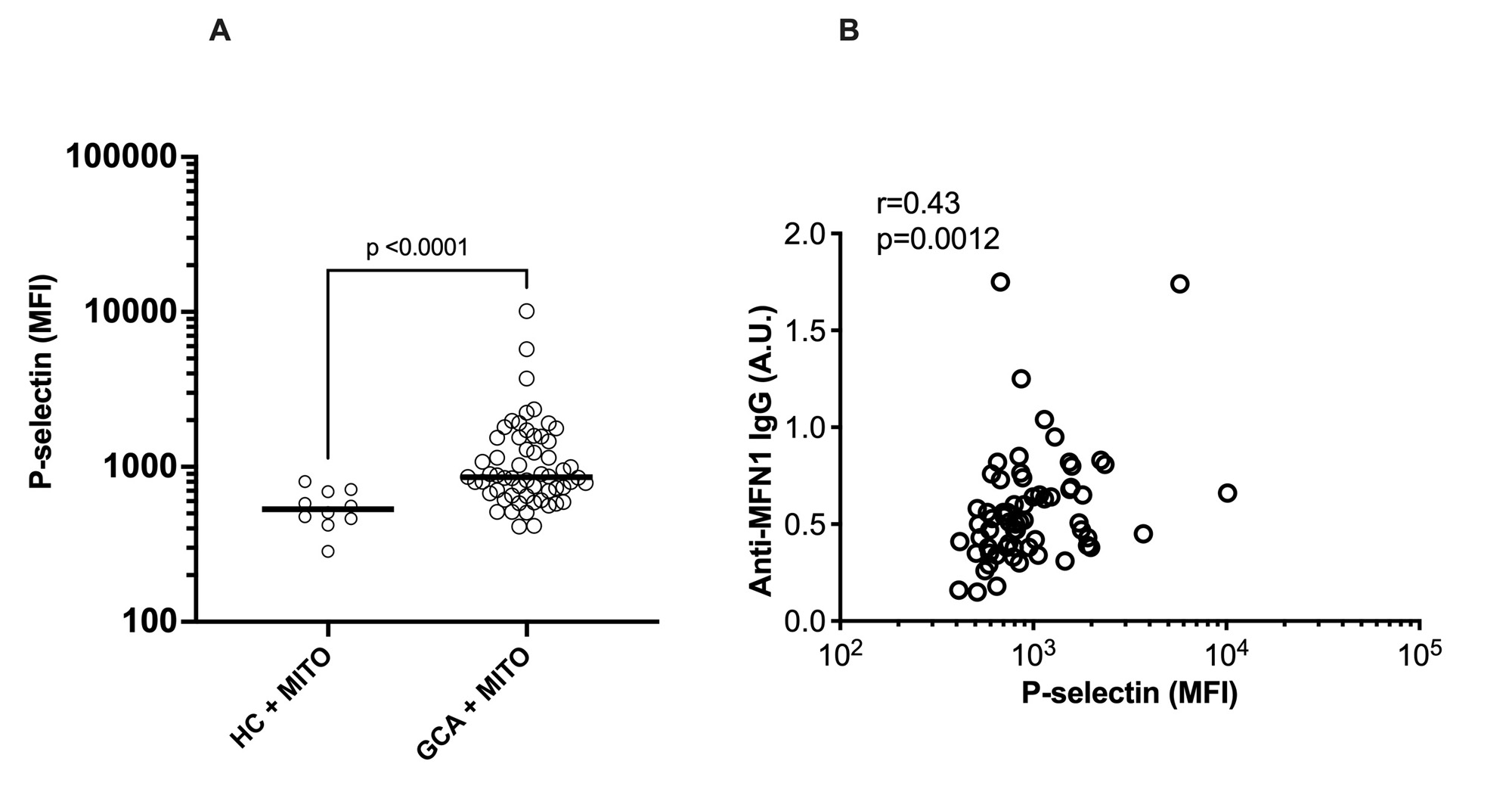
Results: Levels of anti-MFN1 IgG antibodies, a specific AMA, were elevated both in active disease (p< 0.0001) and in remission (p< 0.0001) in patients with GCA (Figure 1A). No differences in levels of anti-MFN1 antibodies were found between patients in remission and active disease for GCA (Figure 1B).No association was observed between anti-MFN1 antibodies and ESR levels (Figure 1C). Though total levels of IgG AMA did not reach statistical significance (p=0.38) in GCA patients compared to HC, IgA AMA levels were significantly higher in patients with GCA as compared to healthy individuals (p=0.003, Figure 2A). Levels of IgA AMA correlated with levels of IgG AMA in patients with GCA (Figure 2B), but not with levels of anti-MFN1 (data not shown). Finally, to investigate a potential pathogenic role of AMAs in GCA, exogenous mitochondria, derived from HepG2 cells, were opsonized with patient plasma and, upon washing, incubated with platelets from healthy individual. Plasma from patients with GCA had enhanced capacity to promote mitochondrial-mediated platelet activation as measured by P-selectin expression on platelet cell surface by flow cytometry, as compared to plasma from HC (p< 0.0001, Figure 3A). Platelet levels of P-selectin were associated with levels of anti-MFN1 IgG in patients with GCA (r=0.43, p=0.0012, Figure 3B).
Conclusion: We report the presence of AMAs in GCA. Presence of autoantibodies targeting mitochondria supports the hypothesis of antigenic mitochondrial components being present in GCA. Our data suggest that mitochondrial targeting by AMAs results in immune complex formation, potently activating platelets, possibly partaking in the thromboembolic morbidity and mortality of the disease. Targeting key drivers of mitochondrial extrusion in GCA could lead to new therapeutic interventions, including suppression of platelet activation.
To cite this abstract in AMA style:
Michailidou D, Grayson P, Hermanson P, Armando Gonzalez-Chapa J, Cuthbertson D, Khalidi N, Koening C, Langford C, McAlear C, Moreland L, Pagnoux C, Seo P, Sreih A, Warrington K, Monach P, Merkel P, Lood C. Role of Mitochondria in Activation of Platelets in Giant Cell Arteritis [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/role-of-mitochondria-in-activation-of-platelets-in-giant-cell-arteritis/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/role-of-mitochondria-in-activation-of-platelets-in-giant-cell-arteritis/