Session Information
Date: Saturday, November 12, 2022
Title: Plenary I
Session Type: Plenary Session
Session Time: 11:30AM-1:00PM
Background/Purpose: The RECITAL trial (NCT01862926) compared rituximab to cyclophosphamide as first line therapy for patients with severe or progressive interstitial lung disease due to idiopathic inflammatory myositis (IIM), systemic sclerosis (SSc) or mixed connective tissue disease (MCTD). At 24 weeks, both rituximab and cyclophosphamide resulted in an improvement in the primary endpoint of FVC and resulted in improvement in measures of quality of life. We analysed the effect of rituximab and cyclophosphamide by CTD sub-group.
Methods: Individuals with ILD related to SSc, IIM or MCTD for whom their caring physician had recommended treatment with cyclophosphamide were enrolled. Subjects were randomised 1:1 to receive cyclophosphamide 600mg/m2 body surface area administered every 4 weeks for 6 doses or rituximab 1g given at baseline and repeated at 2 weeks. A double dummy design ensured blinding of subjects and trial staff throughout the study. The primary endpoint was rate of change in forced vital capacity (FVC) at 24 weeks. Secondary endpoints included safety and tolerability, FVC change at 48 weeks and patient reported quality of life and modified Rodnan skin score in the SSc subset.
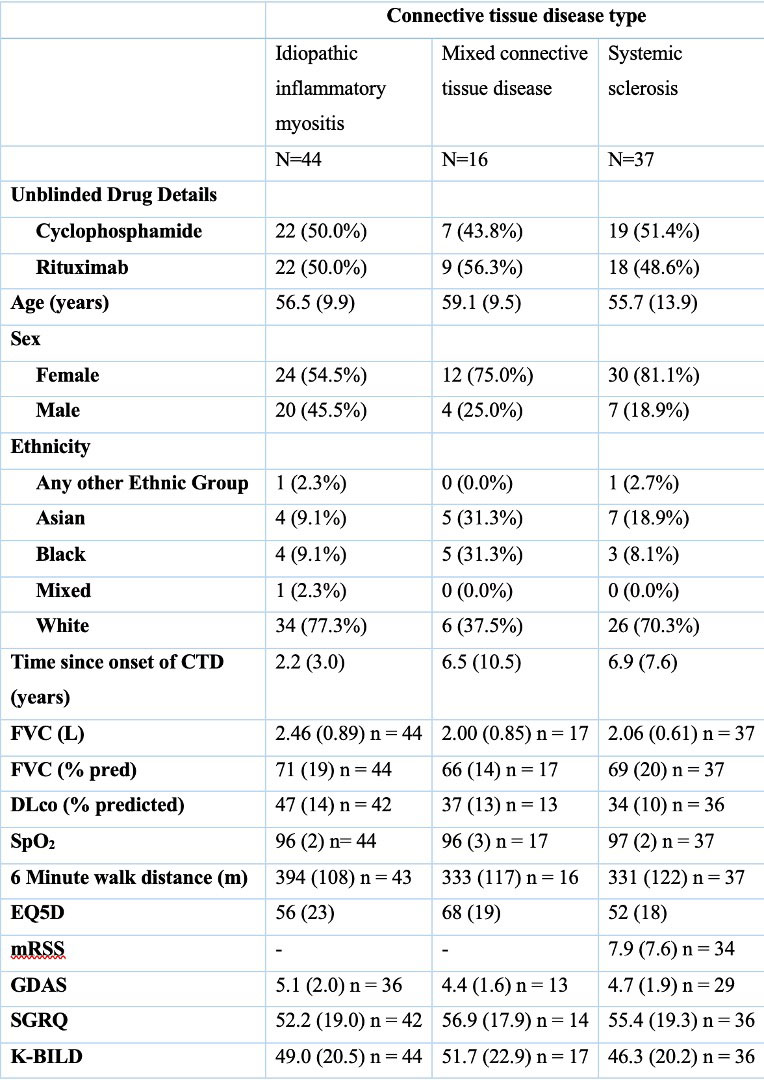
Results: A total of 101 subjects were randomised: 51 to rituximab, 50 to cyclophosphamide. 44 subjects (45.4%) had IIM, 37 (38.1%) had SSc and 16 (16.5%) had MCTD (Table 1). Subjects with MCTD (FVC 66 ± 14 %predicted and a DLco 37 ± 13 % predicted) and scleroderma (FVC 69 ± 20 %predicted and DLco 34 ± 10 %predicted) had greater physiological impairment than the subjects with IIM (FVC 71 ± 19 %predicted and DLco 34 ± 10 % predicted). At 24 weeks, both rituximab and cyclophosphamide resulted in an improvement in FVC (241 mL (95% CI 92.9 – 388.2 mL) and 345.7 mL (198.1 – 493.2 mL) respectively in the IIM subgroup. 24 week change from baseline for MCTD was 208.0 mL (3.3 – 412.7 mL) and 164.8 mL (-5.0 – 379.9 mL) for rituximab and cyclophosphamide whilst for SSc the changes seen were -26.0 mL (-186.8 – 134.6 mL) and -3.3 mL (-154.8 – 148.2 mL) respectively. In the SSc patients there was a slight deterioration in mRss with cyclophosphamide at 24 weeks (1.6 ± 5.7 units) but an improvement with rituximab of -3.4 ± 8.1 units. The difference between the mRSS in the two arms, in an adjusted mixed effects model, was -4.47 units (95% CI -7.99 – -0.95 units, p = 0.013) at 24 weeks in favour of rituximab.
Conclusion: Cyclophosphamide and rituximab had the greatest magnitude of benefit in patients with IIM and MCTD associated ILD. In SSc-ILD therapy stabilised but did not improve lung function. In SSc patients rituximab but not cyclophosphamide improved skin thickness measured by mRss at 24 weeks.
To cite this abstract in AMA style:
Maher T, Tudor V, Saunders P, Gibbons M, Fletcher S, Parfrey H, Denton C, Hoyles R, Renzoni E, Kokosi M, Wells A, Ashby D, Szigeti M, Molyneaux P. Rituximab versus Cyclophosphamide for the Treatment of Connective Tissue Disease Associated Interstitial Lung Disease (RECITAL): A Sub-group Analysis of a Multi-centre Randomised Controlled Trial [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/rituximab-versus-cyclophosphamide-for-the-treatment-of-connective-tissue-disease-associated-interstitial-lung-disease-recital-a-sub-group-analysis-of-a-multi-centre-randomised-controlled-trial/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/rituximab-versus-cyclophosphamide-for-the-treatment-of-connective-tissue-disease-associated-interstitial-lung-disease-recital-a-sub-group-analysis-of-a-multi-centre-randomised-controlled-trial/