Session Information
Date: Sunday, November 8, 2020
Title: Epidemiology & Public Health Poster III: Inflammatory Rheumatic Disease
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Rheumatoid arthritis (RA) increases risk of osteoporosis and fractures. However, limited head-to-head comparative data exists on the risk of non-vertebral osteoporotic fractures (NVFs) among biologic (b) or targeted-synthetic (ts) disease-modifying antirheumatic drugs (b/tsDMARDs). The primary objective of this study was to compare the incidence rate of NVFs in RA patients initiating one of the 9 b/tsDMARDs: abatacept (ABA), adalimumab (ADA), certolizumab (CER), etanercept (ETA), golimumab (GOL), infliximab (INF), rituximab (RIT), tocilizumab (TCZ) or tofacitinib (TOF).
Methods: We analyzed 3 U.S. healthcare claims databases [Optum (2008-03/19), Medicare and MarketScan (2008-17)]. RA patients aged≥18 years who newly started b/tsDMARDs without any use in the prior year were identified. Patients with previous fracture or malignancy were excluded. ADA was the most frequently used drug and selected as a common reference. The primary outcome was a composite endpoint of incident NVFs that included hip, humerus, pelvis and wrist fracture requiring intervention (except pelvis). Secondary outcomes were specific subtypes of fractures. We adjusted for >70 potential confounders including demographics, comorbidities, osteoporotic diagnosis and medication use, prior DMARD use, other medications, and healthcare utilization in each database through propensity score (PS)-based inverse probability treatment weighting. For the as-treated analysis, follow-up time started the day after cohort entry until the first occurrence of: outcome, treatment discontinuation, switching to other b/tsDMARD, nursing home admission, death, disenrollment, or the end of study period. For each drug-comparison, weighted Cox proportional hazards models estimated the hazard ratios (HRs) with 95% CIs, and provided pooled estimates after data source level stratification. Secondary analyses were conducted in patients switching from a tumor necrosis factor inhibitor to a b/tsDMARD.
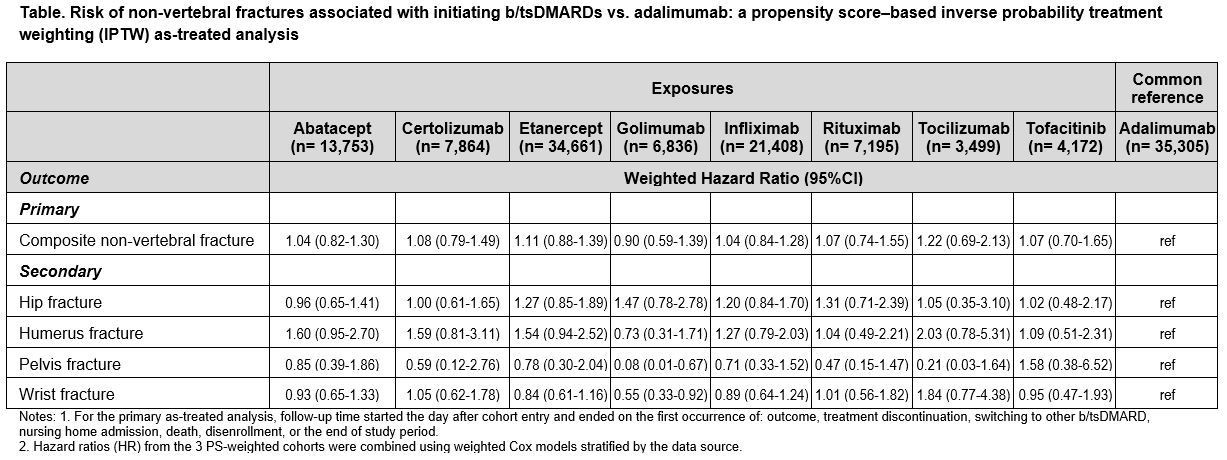
Results: A total of 134,693 b/tsDMARD initiators were identified across 3 databases, of which 26% were ADA initiators. Mean age was 72 years in Medicare, 54 in Optum and 52 in MarketScan. During the 365-day baseline period, 39-65% patients used methotrexate, 64-71% used corticosteroids, and 13-30% had osteoporosis. After PS-weighting, majority of the covariates were well balanced. The average follow-up time (days) ranged from 384 (Optum) to 490 (Medicare). A total of 1,234 NVF events occurred (incidence rate/1,000 person-years: 7.8). The adjusted HRs showed similar risk of composite NVFs in all b/tsDMARD exposures compared to ADA: ABA (HR 1.04, 95% CI 0.82-1.30), CER (1.08, 0.79-1.49), ETA (1.11, 0.88-1.39), GOL (0.90, 0.59-1.39), INF (1.04, 0.84-1.28), RIT (1.07, 0.74-1.55), TCZ (1.22, 0.69-2.13) and TOF (1.07, 0.70-1.65). Consistent findings were observed in the secondary analyses (not shown) and subtypes of NVFs (Table).
Conclusion: This large multi-database cohort study of RA patients found no differences in the risk of composite endpoint of NVFs or specific NVFs after starting ABA, CER, ETA, GOL, INF, RIT, TCZ or TOF versus starting ADA as their first or second b/tsDMARD therapy.
To cite this abstract in AMA style:
Pawar A, Desai R, He M, Bessette L, Kim S. Risk of Non-vertebral Fractures Among Rheumatoid Arthritis Patients Treated with Biologic or Targeted-Synthetic DMARDs: A Multi-Database Comparative Safety Study [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/risk-of-non-vertebral-fractures-among-rheumatoid-arthritis-patients-treated-with-biologic-or-targeted-synthetic-dmards-a-multi-database-comparative-safety-study/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/risk-of-non-vertebral-fractures-among-rheumatoid-arthritis-patients-treated-with-biologic-or-targeted-synthetic-dmards-a-multi-database-comparative-safety-study/