Session Information
Date: Sunday, November 8, 2020
Title: Epidemiology & Public Health Poster III: Inflammatory Rheumatic Disease
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Previous randomized clinical trials and observational studies have signaled an increased risk of skin cancer in rheumatoid arthritis (RA) patients treated with immunosuppressants such as methotrexate (MTX). The objective of this study was to compare the incidence rates of malignant melanoma and nonmelanoma skin cancer (NMSC) in RA patients initiating MTX versus hydroxychloroquine (HCQ) using real-world data.
Methods: Using Medicare claims data (Parts A/B/D 2006-2017), we conducted a cohort study of RA patients who were 65 years or older and initiators of MTX or HCQ as their first DMARD. Cohort entry date was the date of the first dispensing for either MTX or HCQ. At least 365 days of continuous enrollment in Medicare parts A, B, and D was required. We excluded patients who used any disease-modifying antirheumatic drugs (DMARDs), chloroquine, other immunosuppressants, or had a history of malignancy including skin cancer, immunosuppression, psoriatic arthritis, or systemic lupus erythematosus. The primary outcome was a composite of malignant melanoma and NMSC. Malignant melanoma was defined based on a validated algorithm requiring two International Classification of Diseases (ICD) 9 or 10 diagnosis codes separated by 1-60 days (PPV 83%). NMSC was also defined based on a validated algorithm using one ICD 9 or 10 diagnosis code followed by a NMSC-related procedural code occurring within 60 days (PPV 84%). Secondary outcomes were components of the primary outcome: malignant melanoma, NMSC, basal cell carcinoma (BCC), and squamous cell carcinoma (SCC). To control for confounding, we conducted one-to-one propensity score (PS) matching using more than 60 covariates assessed during a 365-day baseline period. We calculated the incidence rates (IRs), hazard ratios (HRs), and corresponding 95% confidence intervals (CIs) for each outcome.
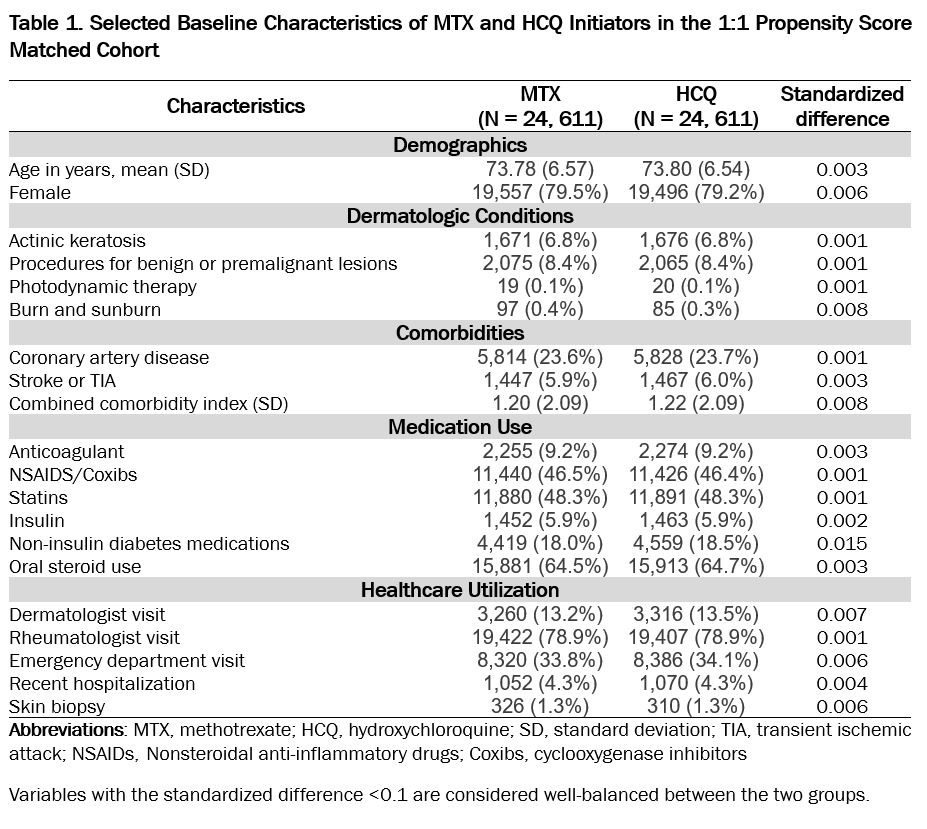
Results: A total of 24,611 PS-matched pairs of MTX and HCQ initiators were identified. The mean (SD) age was 73.8 (6.6) years and 78% were female. Patient demographics, dermatologic conditions, comorbidities, medication use, and healthcare utilization were well-balanced between the two treatment groups at baseline (Table 1). During a total person-years of 52,836, we observed 693 (MTX) and 612 (HCQ) cases of malignant melanoma or NMSC. The IR per 1,000 person-year for the primary outcome was 24.57 (MTX) versus 24.85 (HCQ), and the HR comparing MTX with HCQ was 0.97 (95%CI 0.87-1.09). For secondary outcomes, MTX was associated with an elevated risk of BCC (HR 1.32, 95%CI 1.08-1.60) but a decreased risk of SCC (HR 0.77, 95%CI 0.61-0.97) compared with HCQ (Table 2).
Conclusion: In this large cohort of older RA patients initiating MTX or HCQ as their first DMARD, we found no difference in the risk of skin cancer including malignant melanoma and NMSC. However, for individual subsets of NMSC, the risk of BCC was higher in the MTX group compared with HCQ initiators while the risk of SCC was lower.
To cite this abstract in AMA style:
Lee H, Chen S, Gautam N, Vine S, He M, Desai R, Weinblatt M, Glynn R, Kim S. Risk of Malignant Melanoma and Nonmelanoma Skin Cancer in Rheumatoid Arthritis Patients Initiating Methotrexate versus Hydroxychloroquine [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/risk-of-malignant-melanoma-and-nonmelanoma-skin-cancer-in-rheumatoid-arthritis-patients-initiating-methotrexate-versus-hydroxychloroquine/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/risk-of-malignant-melanoma-and-nonmelanoma-skin-cancer-in-rheumatoid-arthritis-patients-initiating-methotrexate-versus-hydroxychloroquine/