Session Information
Date: Monday, November 18, 2024
Title: Metabolic & Crystal Arthropathies – Basic & Clinical Science Poster III
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Previous studies compared the risk of gout with sodium-glucose cotransporter-2 inhibitors (SGLT2i) versus glucagon-like peptide-1 receptor agonists (GLP1-RA), including exendin-4 based GLP1-RA (exenatide and lixisenatide). Synthetic GLP1-RA (semaglutide, liraglutide and dulaglutide) might be different and have not been well studied in gout. In this study, we examined whether SGLT2i and synthetic GLP1-RA 1) lower the risk of incident gout and 2) lower the risk of cardiovascular events post-gout flares in veterans with type 2 diabetes.
Methods: We conducted an active comparator, new user design study of those initiated for the first-time on insulin glargine, GLP1-RA or SGLT2i between 1/1/2018 to 12/31/2021 in veterans with type 2 diabetes on metformin without a baseline history of gout (N= 142,883). A prescription for these drugs between 1/1/2008 and 1/1/2018 was an exclusion criterion. Follow-up was until 3/31/2023. ICD-10 codes were used to define incident gout and subsequent cardiovascular composite of myocardial infarction, stroke and heart failure within 120 days of incident gout diagnosis. Generalized propensity score-based inverse probability weighting was employed to minimize confounding in Cox regression models for pairwise comparisons of the three drug classes with time to incident gout. A logistic regression model was used to compare the three drug classes and the risk of cardiovascular composite outcome within 120 days of gout diagnosis.
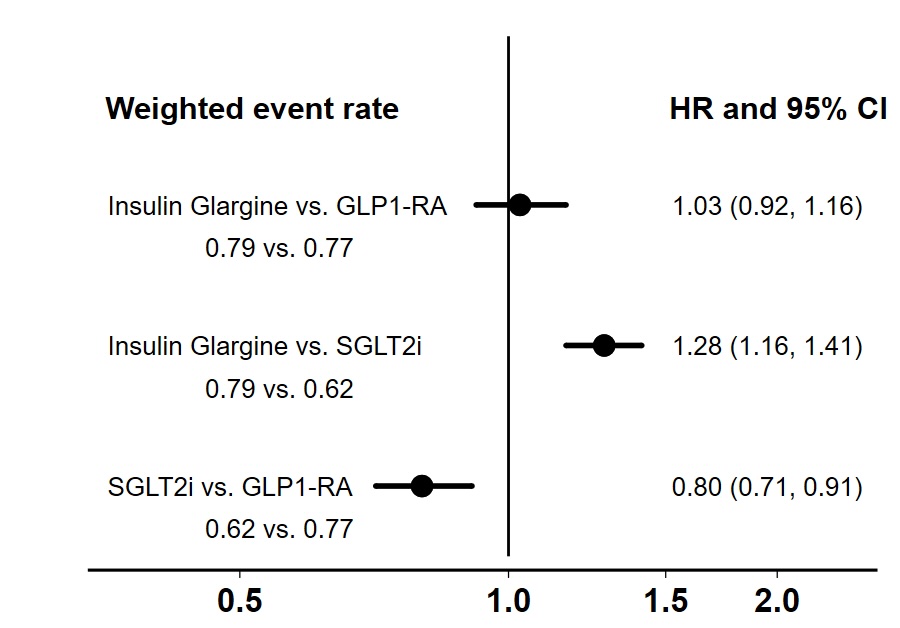
Results: There were 48,588 (34.0%) initiated on insulin glargine, 74,706 (52.3%) on SGLT2i and 19,589 (13.7%) on GLP1-RA. Empagliflozin ( >99%) was the most commonly used SGLT2i. Semaglutide (49.0%), dulaglutide (25.2%) and liraglutide (24.5%) were the commonly used GLP1-RA. There were 2,793 incident gout diagnoses over 391,843 years of follow-up. While insulin glargine and GLP1-RA had similar risk of incident gout, SGLT2i had lower risk compared to either (Figure 1).
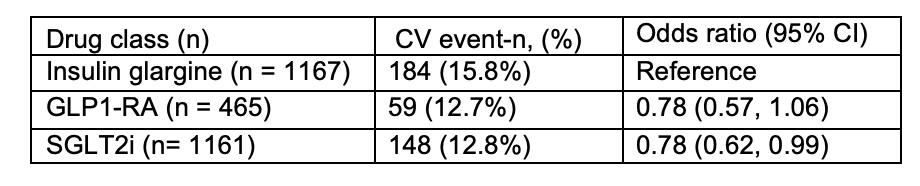
There were 391 (14%) cardiovascular events within 120 days of an incident gout diagnosis. Compared to those who developed gout with initiation on insulin glargine, those who developed gout with initiation on SGLT2i had 22% significantly lower risk of cardiovascular events (Table 1). For similar comparison with insulin glargine, GLP1-RA also lowered the risk by 22% but this was non-significant likely because of the smaller sample size.
Conclusion: SGLT2i lowered the risk of incident gout compared to insulin glargine or GLP1-RA. The risk of cardiovascular events within 120 days of an incident gout diagnosis is very high but this was significantly lowered by SGLT2i and possibly by GLP1-RA.
To cite this abstract in AMA style:
Sarwal A, Takyi A, Wei G, Nevers M, Singh R, Hartsell S, Boucher R, Katkam N, Chakravartula A, Shen J, Beddhu S, Schlesinger N. Risk of Incident Gout and Cardiovascular Events Within 120 Days of Incident Gout with the Use of Insulin Glargine, Glucagon-like Peptide-1 Receptor Agonists, or Sodium-Glucose Cotransporter-2 Inhibitors in Veterans with Type 2 Diabetes [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/risk-of-incident-gout-and-cardiovascular-events-within-120-days-of-incident-gout-with-the-use-of-insulin-glargine-glucagon-like-peptide-1-receptor-agonists-or-sodium-glucose-cotransporter-2-inhibito/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/risk-of-incident-gout-and-cardiovascular-events-within-120-days-of-incident-gout-with-the-use-of-insulin-glargine-glucagon-like-peptide-1-receptor-agonists-or-sodium-glucose-cotransporter-2-inhibito/