Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: With the commercialization of new diagnostic and monitoring tests for rheumatoid arthritis (RA) and an increasing number of advanced practice providers (APPs) in the rheumatology workforce over the past decade, we investigated RA testing patterns by physicians and APPs from 2014 through 2023.
Methods: A retrospective review of RA tests ordered by physicians and APPs in primary care (PC) and rheumatology (RHU) at Labcorp from 2014 – 2023 was conducted. Providers who ordered a yearly average of at least 10 rheumatoid factor (RF) tests, 10 anti-cyclic citrullinated peptide (CCP) tests, and 1 RA panel test were included. Test types included diagnostic tests such as established individual tests (RF, anti-CCP), other individual tests (14.3.3 eta protein, RF isotypes, anti-CarP, anti-CEP-1, anti-Sa, and synovial fluid RF), and panel tests (Rheumatoid Arthritis Profile, RheumAssure, RA Profile Reflex to SeroNeg4, RAdx6 Profile, SeroNeg RAdx4 Profile), and one monitoring test (Vectra®). Medians were used to reduce skewness by providers who ordered an extremely high volume of tests.
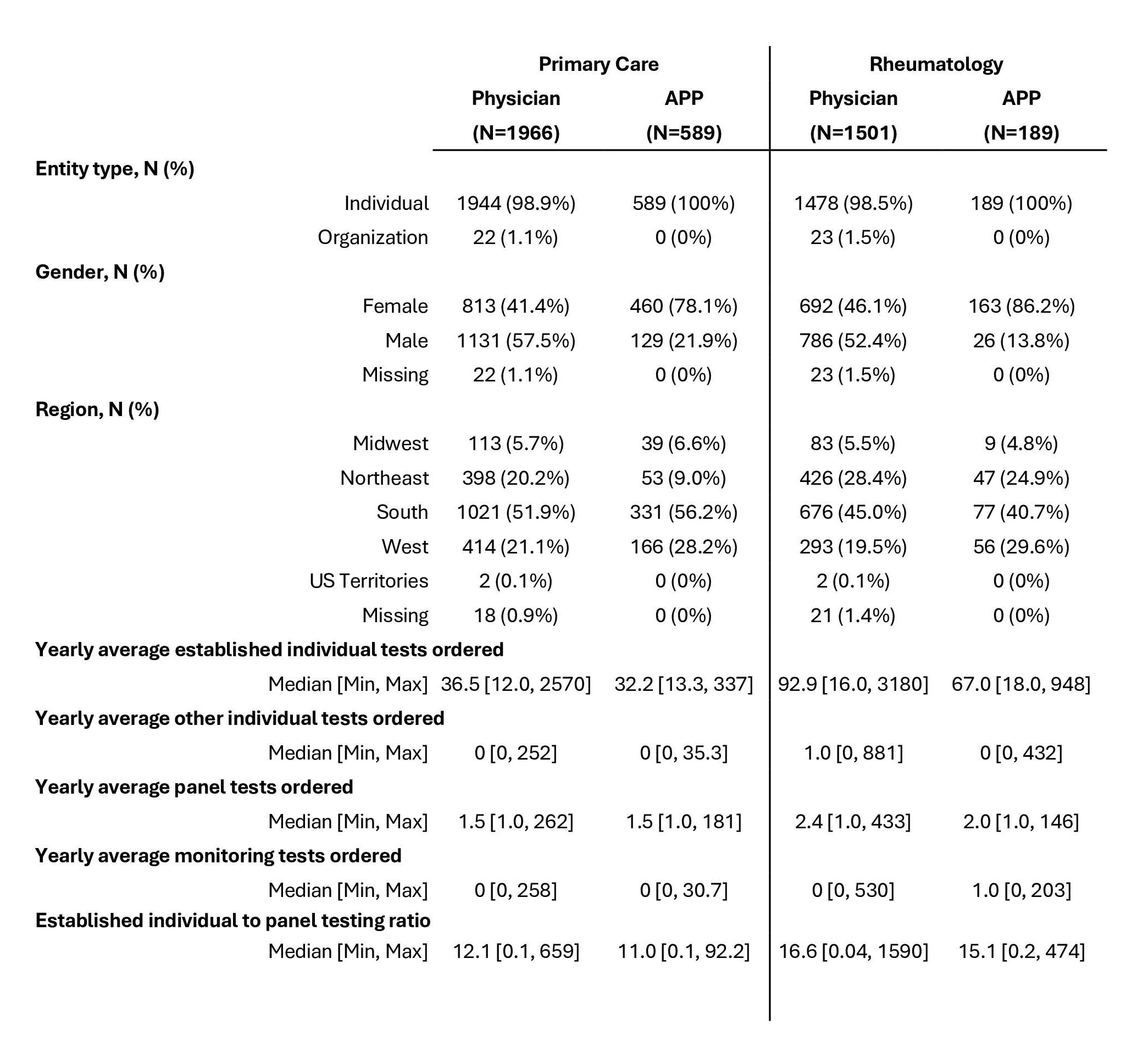
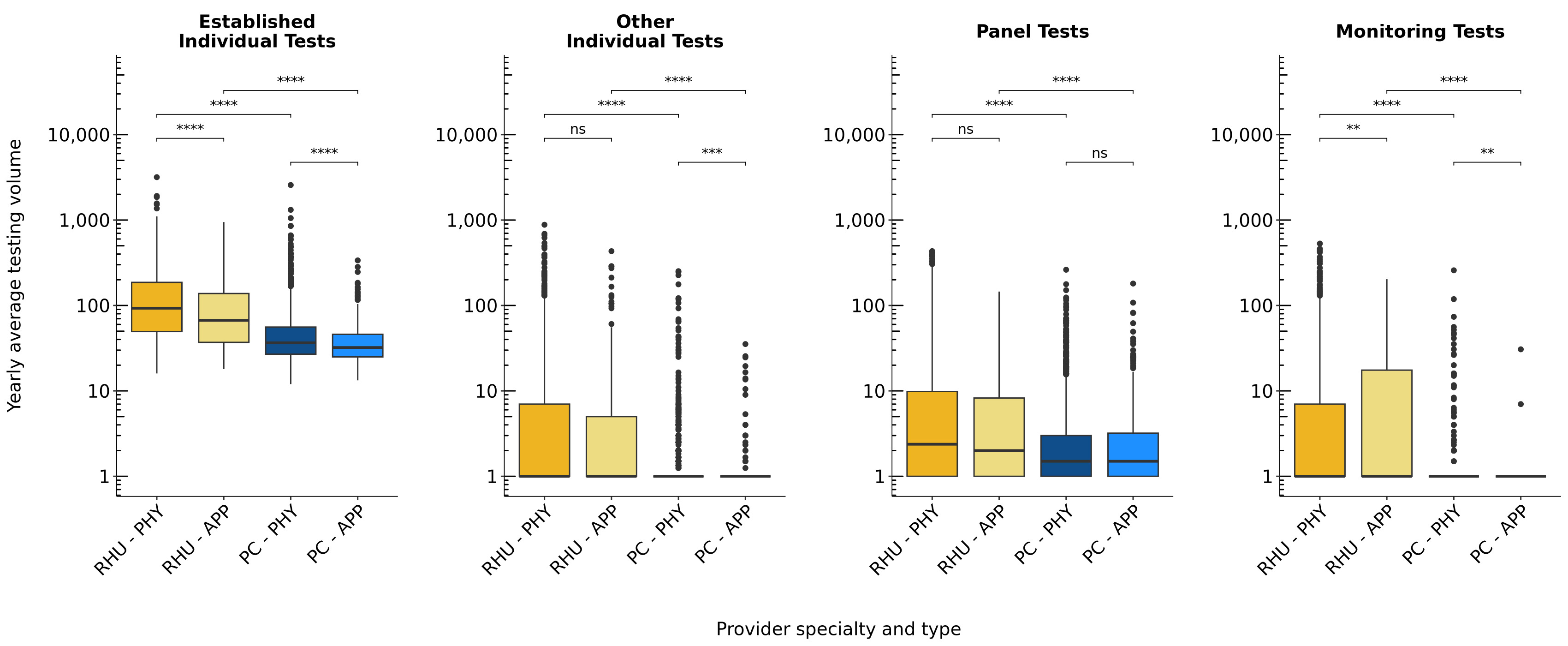
Results: 1,966 PC physicians, 589 PC APPs, 1,501 RHU physicians, and 189 RHU APPs were included (Table 1). For established individual tests: RHU physicians (median: 92.9) ordered significantly higher volumes of RF and anti-CCP compared to RHU APPs (median: 67.0, p < 0.001) and PC physicians (median: 36.5, p < 0.001; Figure 1). PC APPs (median: 32.2) ordered the lowest volumes of RF and anti-CCP. For established individual tests, a median of 16.6 tests were ordered per panel test by RHU physicians, 15.1 by RHU APPs, 12.1 by PC physicians, and 11.0 by PC APPs (Table 1). The volume of yearly average panel tests ordered did not significantly differ between physicians and APPs in RHU (p = 0.25) or PC (p = 0.38). However, RHU physicians (median: 2.4) ordered higher volumes of panel tests compared to PC physicians (median: 1.5, p < 0.001). RHU APPs (median: 2.0) ordered higher volumes of panel tests compared to PC APPs (median: 1.5, p < 0.001). RHU APPs (median: 1.0 [0, 203]) ordered higher volumes of monitoring tests compared to RHU physicians (median: 0.0 [0, 530], p = 0.005) and PC APPs (median: 0.0 [0, 30.7], p < 0.001) on a per capita basis.
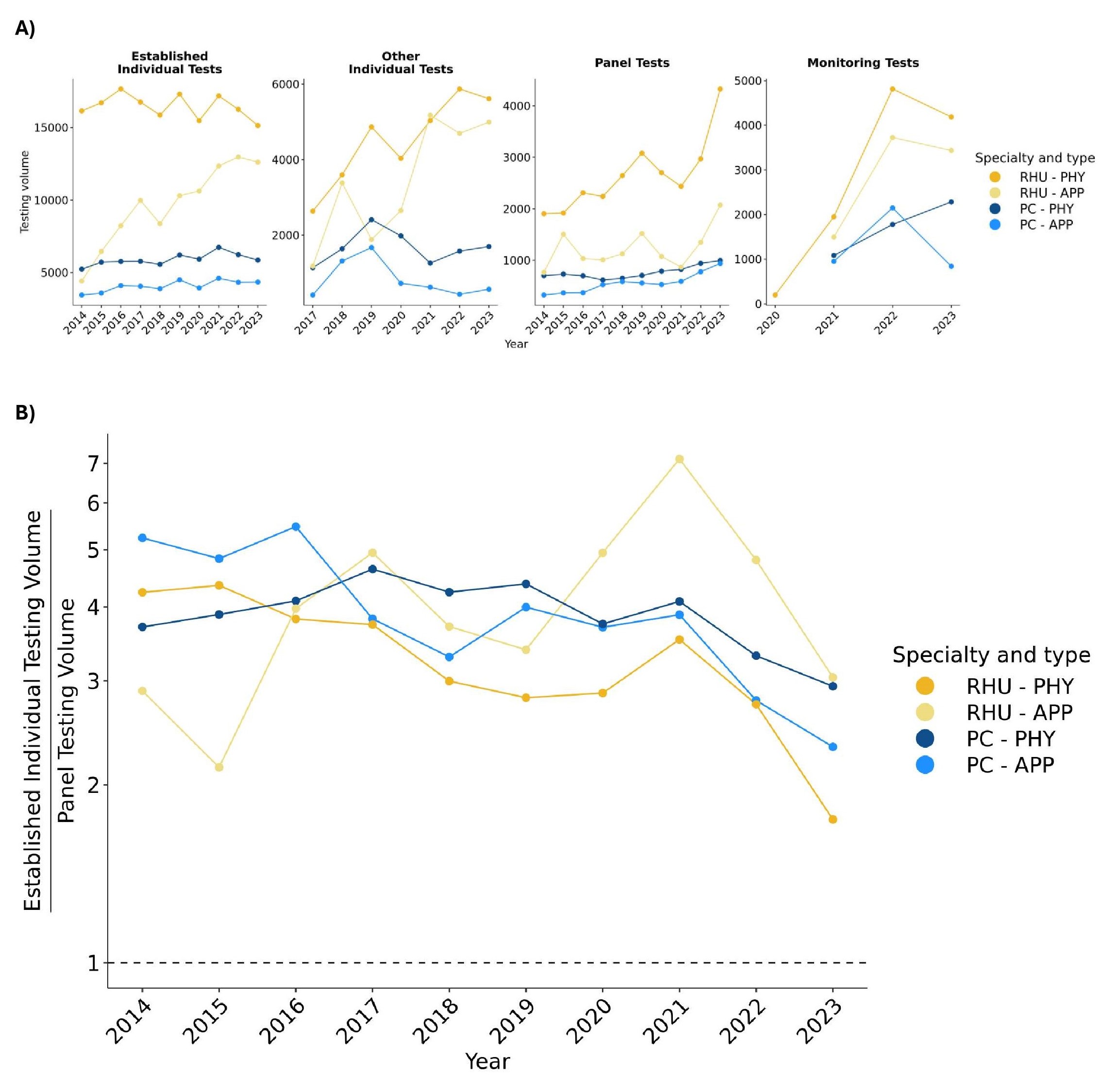
There was a steady increase in the testing volumes of established individual tests from RHU APPs from 2014 – 2023 (Figure 2A). Testing volumes by RHU physicians and RHU APPs for other individual tests increased from 2017 – 2023. An increase in testing volumes of monitoring tests from all provider groups except for PC APPs from 2021 – 2023 was also noted. Finally, the ordering of panel tests compared to established individual tests, i.e. RF and anti-CCP, was increased in all provider groups from 2021 – 2023 (Figure 2B).
Conclusion: RHU physicians ordered the most RA diagnostic tests. RA diagnostic tests ordered by RHU APPs have steadily increased from 2014 – 2023. Higher volumes of RA monitoring tests on a per capita basis ordered by RHU APPs compared to RHU physicians may reflect the former’s role in patient follow-up visits. With the introduction of new diagnostic test panels in 2021, panel test utilization has subsequently increased compared to established individual tests.
To cite this abstract in AMA style:
Lee K, Lee M, Alfego D, Clark K, Naides S. Rheumatoid Arthritis Testing Patterns by Physicians and Advanced Practice Providers from a National Commercial Laboratory, 2014 – 2023 [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/rheumatoid-arthritis-testing-patterns-by-physicians-and-advanced-practice-providers-from-a-national-commercial-laboratory-2014-2023/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/rheumatoid-arthritis-testing-patterns-by-physicians-and-advanced-practice-providers-from-a-national-commercial-laboratory-2014-2023/