Session Information
Session Type: Abstract Session
Session Time: 1:00PM-2:30PM
Background/Purpose: In the rheumatoid arthritis (RA) joint, resident synovial fibroblasts (FLS) interact with infiltrating leukocytes. We previously identified a population of sublining FLS responsive to IFNg and TNF, likely originating from activated T cells, that also express HLA-DR, suggesting a role for direct interactions between FLS and T cells. Here we investigated how FLS acting as antigen presenting cells (APC) drive divergent T cell activation and polarization states as compared to professional APC (macrophages).
Methods: FLS were dissociated from synovial tissue obtained from a prospective cohort of RA patients undergoing arthroplasty (HSS IRB 2014-233) and used at passages 3-6. We used superantigen staphylococcal enterotoxin B (SEB) to drive HLA-DR dependent T cell activation by either FLS or macrophages as APC. FLS and healthy donor (HD) or RA macrophages differentiated from blood monocytes (CD14+) were cultured alone or in combination with HD or RA T cells (CD3+) and activated with SEB (1 ng/mL). T cell activation and proliferation were measured via flow cytometry. T cell gene expression was measured by bulk RNA sequencing.
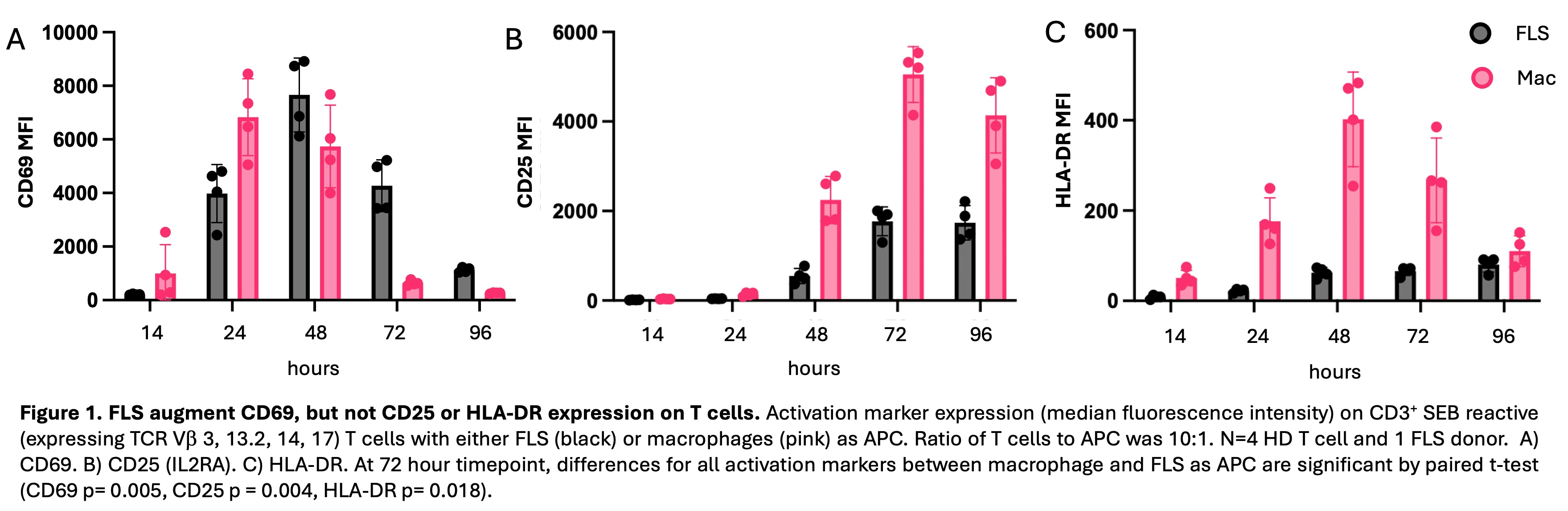
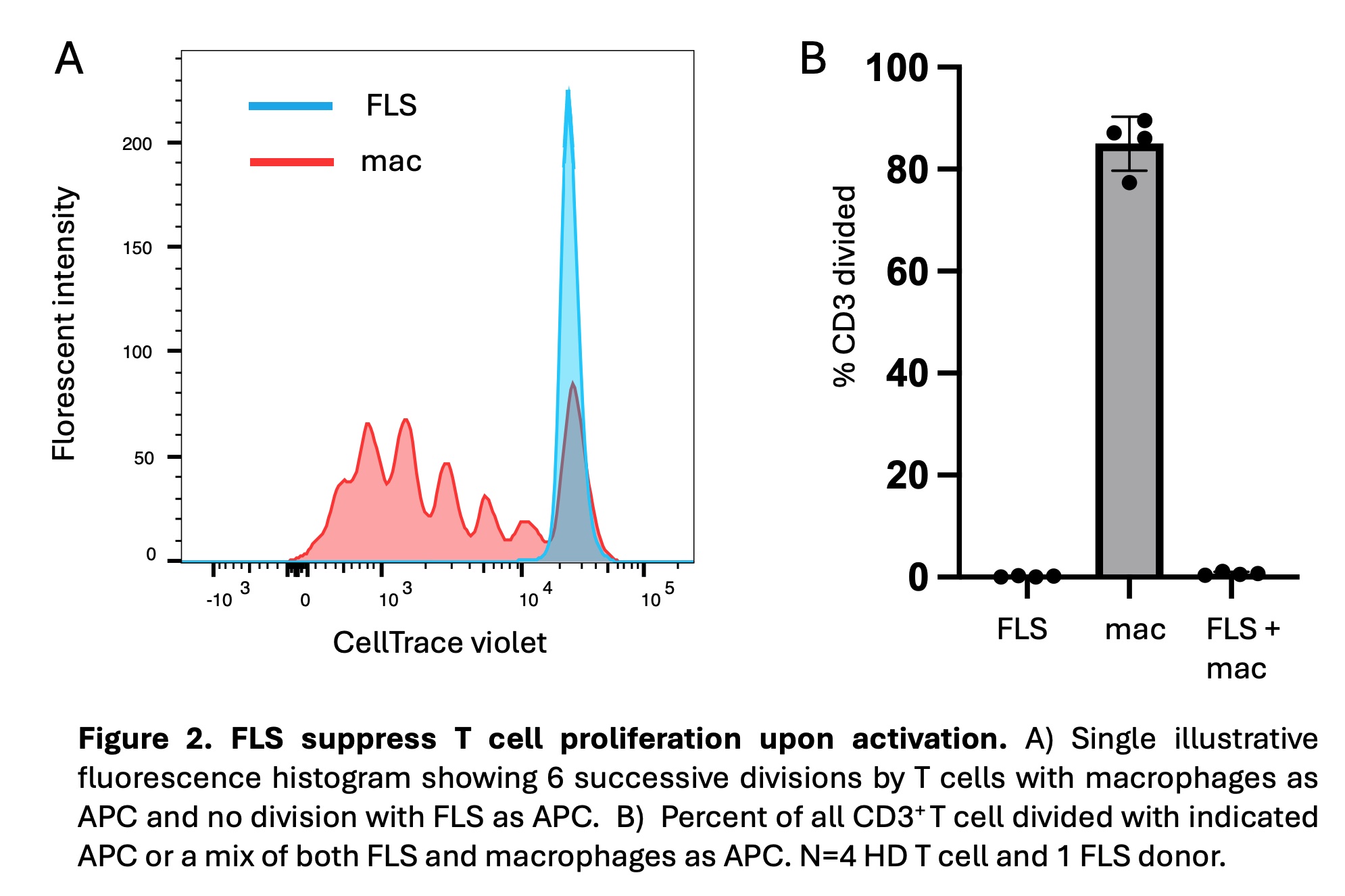
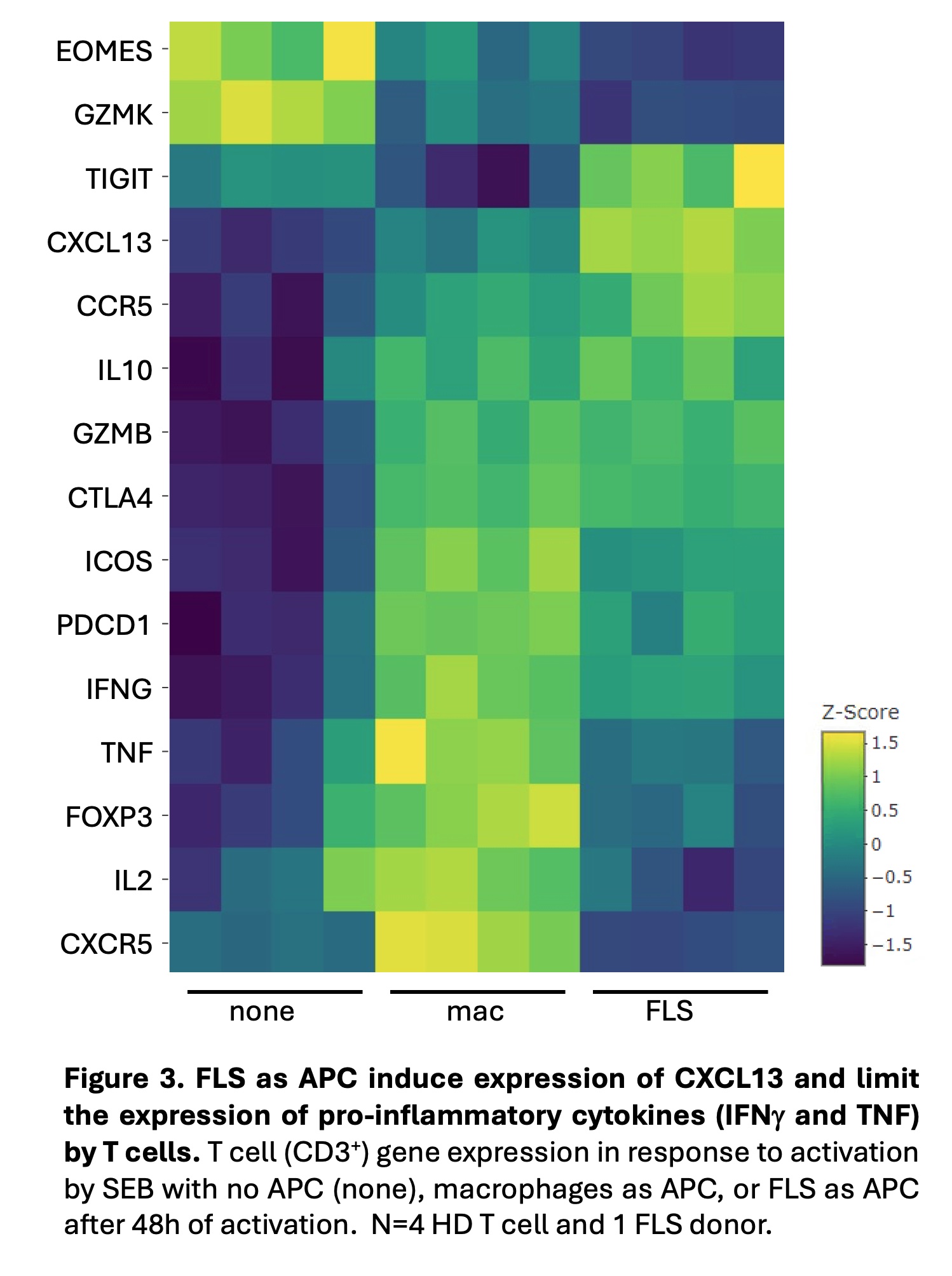
Results: T cells activated by SEB with FLS, as compared to macrophages, as APC exhibited enhanced and prolonged CD69 expression beginning at 48 hours of activation (Fig. 1). T cells activated with macrophages as APC expressed higher levels of CD25 and HLA-DR. These differences were all significant at 72 hours. FLS exerted a larger effect on CD69 expression at 72 hours in memory (CD45RA–) as compared to naïve (CD45RA+CCR7+) T cells. This effect was not due to an increase in FOXP3+ regulatory T cells, as intracellular FOXP3 expression was lower with FLS as APC. Similar results were seen with T cells derived from RA patient PBMCs and synovia. FLS as APC suppressed T cell proliferation following activation (Fig. 2). FLS-driven proliferation suppression was dominant over proliferation induced by macrophages. T cells activated by FLS, as compared to macrophages, as APC expressed less IL2 and pro-inflammatory cytokines (TNF, IFNG), and more IL10 as well as TIGIT, both negative regulators of inflammation (Fig. 3). Increased expression of CXCL13 and decreased expression of CXCR5 with FLS as APC raises the possibility of FLS driving T cells towards a T peripheral helper phenotype.
Conclusion: Compared to macrophages as professional APC, FLS enhance and prolong T cell CD69 expression, which plays a role in the negative regulation of activation. In addition, FLS limit the expression of additional T cell activation makers, suppress proliferation, and constrain pro-inflammatory cytokine expression. Our work supports the likelihood that antigen presentation by resident FLS can suppress infiltrating T cells and raises the question as to whether loss of this peripheral tolerance mechanism contributes to RA pathogenesis.
To cite this abstract in AMA style:
Romoff M, Ramirez D, Dicarlo E, Goodman S, Rudenska A, Donlin L, Smith M. Rheumatoid Arthritis Synovial Fibroblast Modulation of T Cell Activation [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/rheumatoid-arthritis-synovial-fibroblast-modulation-of-t-cell-activation/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/rheumatoid-arthritis-synovial-fibroblast-modulation-of-t-cell-activation/