Session Information
Session Type: Abstract Session
Session Time: 3:45PM-4:00PM
Background/Purpose: Intra-articular corticosteroids are widely used for routine management of chronic pain from knee osteoarthritis (KOA), though estimates of their benefit vary widely. We aimed to quantify the short-term benefit of corticosteroid injections in reducing symptoms of KOA in a large, pragmatic, multi-site clinical trial.
Methods: This was a factorially-designed crossover randomized trial among patients with KOA across 4 Veteran Affairs Medical Centers. Participants were 40-80 years of age, had a clinical diagnosis of KOA, a Kellgren-Lawrence X-ray grade > or = 1, and a clinical indication for joint injection according to the treating provider. Participants were randomized to an incentive arm to promote physical activity, or an attention control. Within these arms, participants were further randomized to receive blinded injections with corticosteroid plus lidocaine (40 mg methylprednisolone) or lidocaine-only in a crossover design over 28 weeks. The primary outcome was the change in the Knee Osteoarthritis Outcome Score (KOOS) (range 0-100). A change of >10 was considered the minimal clinically important difference (MCID). Linear or generalized mixed-effects models were used to evaluate the effect of the corticosteroid injection over all outcomes between 2-12 weeks. We also performed a crossover analysis among 196 participants that received a second injection at week 16 (Nf392). Sensitivity analyses included a last-observation-carried-forward approach to account for missingness as well as sub-group analyses.
Results: Of 231 enrolled, 221 were randomized (mean age 64 years; 84% male, median K-L grade of 3). 176 (80%) had received a prior injection. Those randomized to initial corticosteroids showed no improvement in total KOOS scores compared to lidocaine-only at any time point or when evaluating all outcomes over 12-weeks [B: 1.72 (95% CI: -1.08, 4.52) p=0.23] (Figure 1a, Table 1). The probability of achieving KOOS MCID was 20.9% v. 16.8% for the lidocaine-only arm (Risk Difference [RD] = 0.041 (95% CI: -4.1, 12.3); Number Needed to Treat [NNT] = 24 (95% CI: 8, ∞). A crossover analysis comparing 196 participants to themselves demonstrated no benefit over 12-weeks [B: 0.36 (95% CI: -1.17, 1.90) p=0.29] (Figure 1b). The probability of achieving MCID was 19.7% v. 15.3% for lidocaine-only (RD=0.044 [95% CI: -0.011, 0.10]; NNT=23 [95% CI: 10, ∞]). There were no carryover effects. Results were similar for secondary outcomes, in sensitivity analysis, and across subgroups (Table 1; Figure 2). There was no interaction with the exercise incentive arm. Among those receiving both injections, only 55% correctly guessed the order of their injections. Rescue injections were infrequent (n=6 during lidocaine-only; n=7 during corticosteroid).
Conclusion: In this well-powered, blinded, pragmatic, randomized trial, corticosteroid injections provided no significant benefit over 12 weeks compared to lidocaine-only injections. These findings, in the context of the existing literature, suggest limited benefit of this commonly performed procedure in real-world practice and raise new questions about whether this intervention should be widely used for routine management of KOA symptoms.
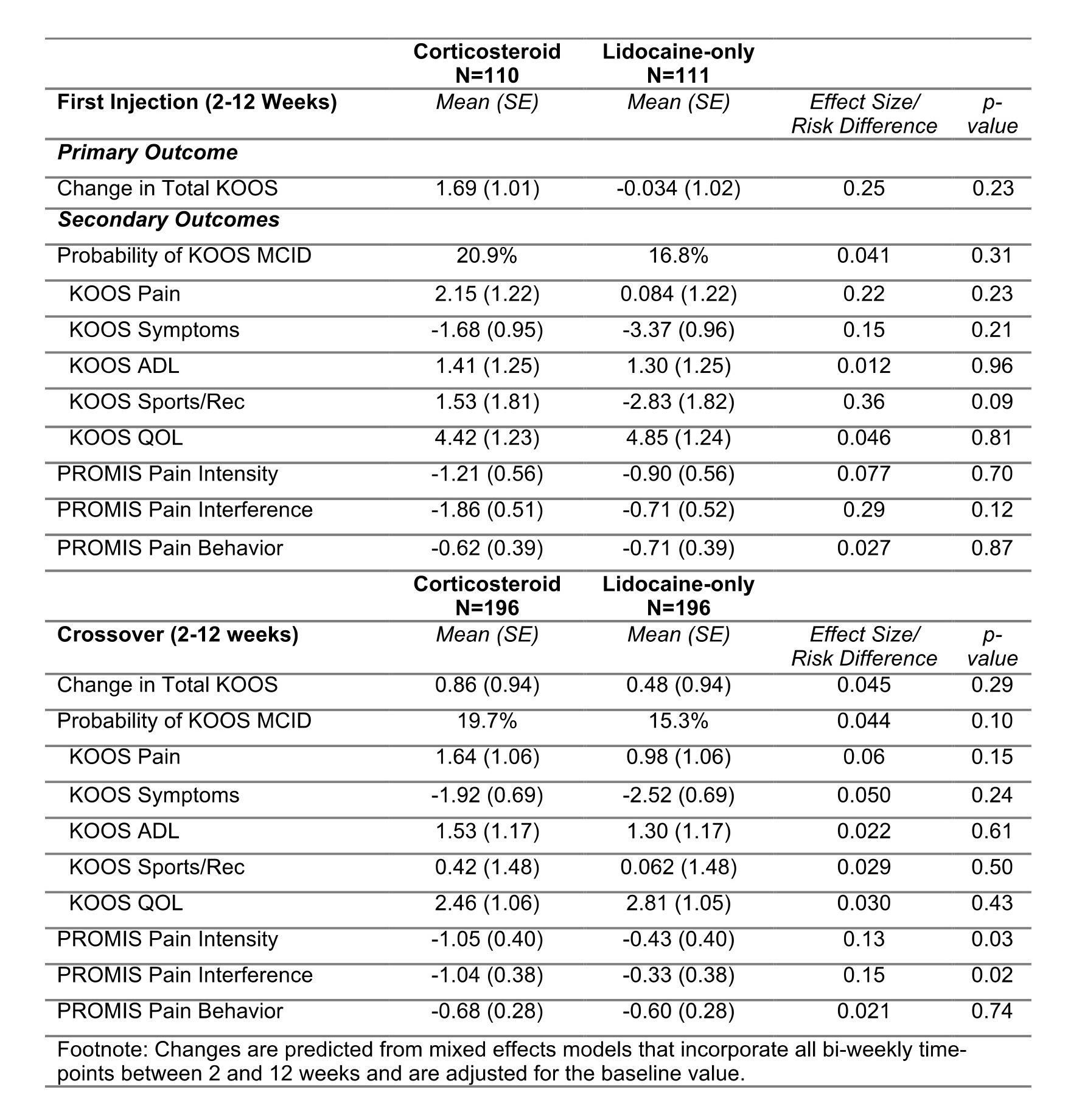 Figure 1: Changes in total KOOS (with 95% confidence intervals) in the corticosteroid plus lidocaine (purple) and lidocaine-only (green) arms over 12 weeks of follow up for A) the primary analysis between weeks 0-12, and B) the crossover analysis. An increase in KOOS indicates an improvement/reduction in KOA symptoms.
Figure 1: Changes in total KOOS (with 95% confidence intervals) in the corticosteroid plus lidocaine (purple) and lidocaine-only (green) arms over 12 weeks of follow up for A) the primary analysis between weeks 0-12, and B) the crossover analysis. An increase in KOOS indicates an improvement/reduction in KOA symptoms.
.jpg) Table 1: Time-averaged change from baseline among those receiving corticosteroid plus lidocaine compared to those receiving lidocaine only over all time-points between 2 and 12 weeks. The standardized effect size (or risk difference for binary outcomes) are also shown.
Table 1: Time-averaged change from baseline among those receiving corticosteroid plus lidocaine compared to those receiving lidocaine only over all time-points between 2 and 12 weeks. The standardized effect size (or risk difference for binary outcomes) are also shown.
.jpg) Figure 2: Subgroup analyses for primary outcome comparing corticosteroid plus lidocaine to lidocaine-only. Point estimates and confidence intervals shown are the estimates of difference betweenc corticosteroid and lidocaine-only groups from time-averaged mixed-effects models within each subgroup of interest. Abbreviations: KL, Kellgren-Lawrence; KOOS, Knee Osteoarthritis Outcome Score; LOCF, last observation carried forward.
Figure 2: Subgroup analyses for primary outcome comparing corticosteroid plus lidocaine to lidocaine-only. Point estimates and confidence intervals shown are the estimates of difference betweenc corticosteroid and lidocaine-only groups from time-averaged mixed-effects models within each subgroup of interest. Abbreviations: KL, Kellgren-Lawrence; KOOS, Knee Osteoarthritis Outcome Score; LOCF, last observation carried forward.
To cite this abstract in AMA style:
Baker J, Wysham K, Quinones M, England B, Jin K, Olave M, Wetzel S, Gillcrist R, Lavery C, Keller N, Hayes K, Kramer B, Brubeck H, Ateh B, White D, Ogdie A, Xiao R, Neogi T, Scanzello C. Results of Large Multi-Site Pragmatic Clinical Trial Comparing Corticosteroids or Blinded Lidocaine-only Injections in Treating Osteoarthritis of the Knee [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/results-of-large-multi-site-pragmatic-clinical-trial-comparing-corticosteroids-or-blinded-lidocaine-only-injections-in-treating-osteoarthritis-of-the-knee/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/results-of-large-multi-site-pragmatic-clinical-trial-comparing-corticosteroids-or-blinded-lidocaine-only-injections-in-treating-osteoarthritis-of-the-knee/
