Session Information
Date: Tuesday, November 9, 2021
Title: Pediatric Rheumatology – Clinical Poster III: Miscellaneous Rheumatic Disease (1614–1644)
Session Type: Poster Session D
Session Time: 8:30AM-10:30AM
Background/Purpose: Children with uveitis often require topical eye medications (eye drops) to control inflammation (glucocorticoids) and complications such as elevated intraocular pressure and synechiae (glaucoma or cycloplegic agents). Eye drops may sting when instilled and be given frequently during the day, which may impact quality of life (QOL) and function. The Effects of Youngsters’ Eyesight on QOL (EYE-Q) is a valid pediatric uveitis-focused questionnaire that assesses vision-related QOL (VRQOL) and vision-related function (VRF). The Pediatric Quality of Life Inventory (PedsQL) questionnaire assesses health-related QOL (HRQOL). The purpose of this study was to assess the responsiveness of the EYE-Q and PedsQL to changes in topical medication regimen in children with non-infectious uveitis (NIU).
Methods: Pediatric patients from a tertiary medical care center with a diagnosis of NIU were enrolled. EYE-Q and PedsQL questionnaires were administered to both patients and parent-proxies at baseline and at the 6-month visit. Ophthalmologic examination findings and current treatments were collected. Two comparison groups were created to assess the sensitivity of the EYE-Q based on topical eye medication regimen: Group 1 patients had changes in either type of topical medication or in the frequency of drops between baseline and follow-up. Group 2 patients did not have any changes in topical eye medication regimen. Repeated-measures mixed-models was used to examine changes in scores for both groups. An alpha of p < 0.05 was considered statistically significant.
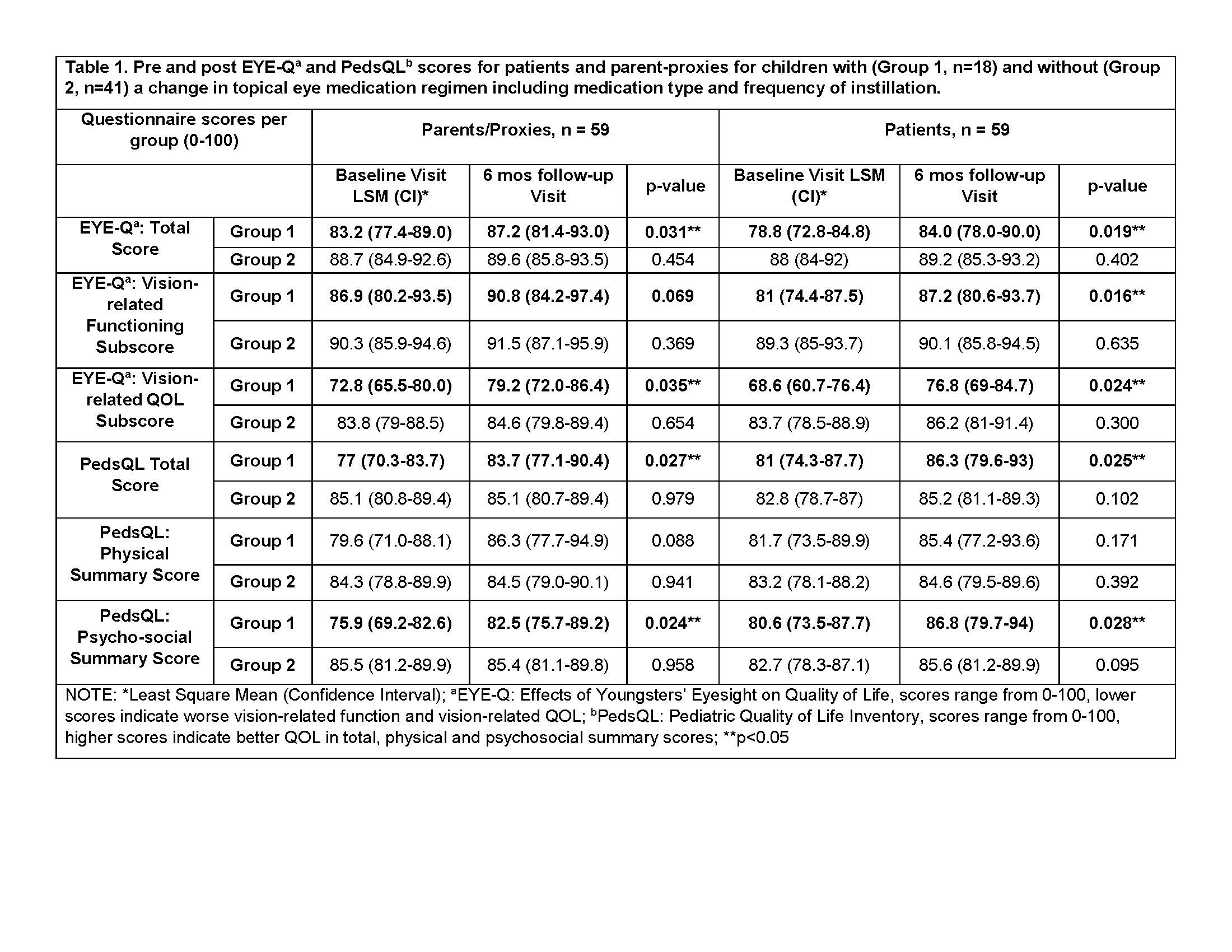
Results: Fifty-nine pediatric patients/parent-proxies participated in the study. Average patient age was 11.1 years (SD=3.4); most were White (85%) and female (70%). 59% had a diagnosis of JIA and uveitis, while 41% had uveitis-only; 18 patients had a change in their topical eye medication regimen (Group 1). Within Group 1, EYE-Q Total scores significantly increased from baseline to 6-month visit (patients, 79 vs. 84; parents 83 vs. 87, p=0.03) (Table 1). Group 1 patients also reported a significant improvement in VRF (81 vs. 87, p=0.02). The PedsQL Total and psychosocial summary scores also increased significantly for patients (81 v. 86, p=0.03; 81 v. 87, p=0.03) and parents (77 v. 84, p= 0.03; 76 v. 83, p=0.02) respectively (Table 1). There were no significant changes in EYE-Q or PedsQL score for Group 2. Despite improved VRQOL scores, Group 1 had significantly lower VRQOL compared to Group 2 (77 vs. 86, p< 0.05) at the 6-month visit.
Conclusion: The EYE-Q and PedsQL were sensitive to clinically meaningful differences in QOL in children with NIU who required changes in treatment regimen. A change in topical medications or the frequency of administration of eye drops led to improved QOL and function. Interestingly, children without changes in topical therapy had similar QOL and function at both time points. Improved scores from baseline to 6-month follow-up exam may be attributed to disease stabilization or adjustment to the implemented changes over time. Further study on the impact of topical eye drops on a child’s QOL and function is needed.
To cite this abstract in AMA style:
Miraldi Utz V, Cassedy A, Hennard T, Mwase N, Angeles-Han S. Responsiveness of Quality of Life and Function Assessment to Changes in Topical Eye Medications in Children with Uveitis [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/responsiveness-of-quality-of-life-and-function-assessment-to-changes-in-topical-eye-medications-in-children-with-uveitis/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/responsiveness-of-quality-of-life-and-function-assessment-to-changes-in-topical-eye-medications-in-children-with-uveitis/