Session Information
Date: Monday, October 27, 2025
Title: (0934–0954) Systemic Lupus Erythematosus – Animal Models Poster
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Lupus nephritis (LN) is a severe manifestation of SLE that can progress to renal fibrosis, and eventual renal failure. In LN, tubulointerstitial inflammation and macrophage infiltration are associated with a poor prognosis. Alongside macrophages, the renal fibrotic niche contains fibroblasts, and endothelial cells; however, the mechanisms of their crosstalk remain poorly understood. Using spatial transcriptomics in LN mouse models and single-cell analysis of in vitro cocultures, we reveal macrophage-driven fibroblast activation as a potential driving feature of renal fibrosis in LN.
Methods: Spatial transcriptomics in NZB/W F1 mice was performed using the 10x Genomics Xenium 389 gene platform, with markers for cell interior and boundaries and an additional gene panel customized to identify high-resolution myeloid populations. Primary NZB/W mouse embryonic fibroblasts (MEFs) were cocultured with FACS-sorted renal resident macrophages (RMs) from nephritic and pre-nephritic NZB/W mice. Cocultures were analyzed by flow cytometry and scRNA-seq. Data were processed using Seurat and Harmony, and cell-cell communication was inferred using CellChat. MEF scratch wound assays with conditioned media (CM) from cocultures were performed, followed by bulk RNA-seq and GSEA for pathway analysis.
Results: Resident macrophages are located in the interstitium and acquire a damage associated Spp1+ gene expression profile during nephritis. In nephritic mice, resident macrophages, and not infiltrating macrophages, preferentially colocalized with FAP⁺ fibroblasts in renal infiltrates and peritubular spaces (Fig. 1A-B), correlating with areas of renal damage and fibrosis. In NZB/W MEF-RM cocultures, flow cytometry and scRNA-seq revealed Spp1+ and Spp1- macrophage populations and a heterogenous expansion of fibroblast populations, including Fap⁺ and Periostin⁺ MEFs, enriched for pro-fibrotic and inflammatory gene signatures (Fig. 2A). CellChat analysis identified signaling from Spp1⁺ macrophages to Fap⁺ MEFs via IL-1, TNF, and OSM, and GRN (Fig. 2B), facilitating a tissue remodeling phenotype. CM from nephritic cocultures induced a pro-inflammatory transcriptional program in MEFs, characterized by elevated Cxcl1, Cxcl5, Cxcl14, and Hif1a, confirmed by GSEA (Fig. 2C). Nephritic CM-treated MEFs experienced impaired wound closure in a scratch assay after 18 hours (Fig. 2D). In a separate study, we demonstrated that mouse and human LN resident kidney macrophages display similar transcriptional profiles and occupy comparable locations within the kidney. This similarity suggests that our findings in mouse models may also be applicable to human LN.
Conclusion: Spp1⁺ resident macrophages in nephritic kidneys localize with and drive fibroblast activation and reprogramming through direct interaction and soluble signaling. Direct macrophage signaling via IL-1, TNF, OSM, and GRN promotes both pro-fibrotic and inflammatory fibroblasts, while indirect signaling induces a pro-inflammatory signature and impairs fibroblast wound healing. Together, these results identify potential targets to modulate renal fibrosis and improve outcomes of LN.
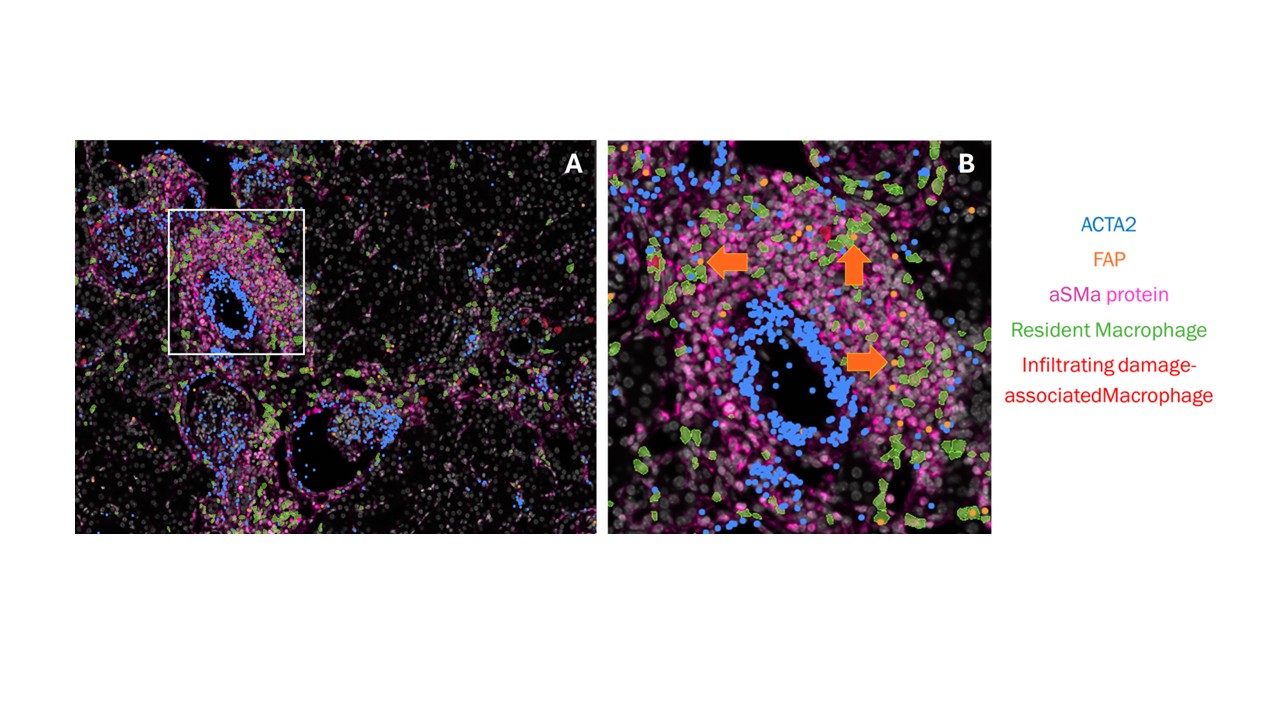 Figure 1: Spatial transcriptomic representation of a nephritic NZB/W kidney. A. An overview of a blood vessel immune infiltrate with resident macrophages labeled in green polygons and infiltrating damage-associated macrophages labeled in red. mRNA transcripts of ACTA2 and FAP are labeled with blue and orange dots, respectively. aSMA protein is depicted in pink. B. An enlarged image of the vessel infiltrate boxed in white in (A), showing resident macrophages colocalized with FAP+ ACTA2+ fibroblasts within the infiltrate.
Figure 1: Spatial transcriptomic representation of a nephritic NZB/W kidney. A. An overview of a blood vessel immune infiltrate with resident macrophages labeled in green polygons and infiltrating damage-associated macrophages labeled in red. mRNA transcripts of ACTA2 and FAP are labeled with blue and orange dots, respectively. aSMA protein is depicted in pink. B. An enlarged image of the vessel infiltrate boxed in white in (A), showing resident macrophages colocalized with FAP+ ACTA2+ fibroblasts within the infiltrate.
.jpg) Figure 2: Direction and indirect fibroblast activation in vitro. A. UMAP of 6 MEF populations and 2 NZB/W RM populations from 48-hour direct contact coculture. N = 10, 3 nephritic mice RM-MEF cocultures, 3 pre-nephritic mice RM-MEF cocultures, 2 Time 0 MEF cultures, and 2 Time 48 cultures. B. Chord diagram of CellChat analysis of direct contact coculture of NZB/W nephritic and pre-nephritic RMs with MEFs. The thickness of each chord represents the magnitude of interaction. Blue chords originate from SPP1+ macrophages, brown from SPP1- macrophages, and red from FAP+ fibroblasts. C. GSEA plot of GOBP_Inflammatory Response pathway. Enrichment of the Inflammatory response is seen in the MEFS treated with the nephritic RM-MEF CM compared to the prenephritic (Young) RM-MEF CM. D. 18-hour scratch wound analysis of MEFs treated with conditioned media from the respective cocultures. Three biological replicates for each condition performed in duplicates. *p < 0.05 between MEFs treated with prenephritic and nephritic RM-MEF coculture CM.
Figure 2: Direction and indirect fibroblast activation in vitro. A. UMAP of 6 MEF populations and 2 NZB/W RM populations from 48-hour direct contact coculture. N = 10, 3 nephritic mice RM-MEF cocultures, 3 pre-nephritic mice RM-MEF cocultures, 2 Time 0 MEF cultures, and 2 Time 48 cultures. B. Chord diagram of CellChat analysis of direct contact coculture of NZB/W nephritic and pre-nephritic RMs with MEFs. The thickness of each chord represents the magnitude of interaction. Blue chords originate from SPP1+ macrophages, brown from SPP1- macrophages, and red from FAP+ fibroblasts. C. GSEA plot of GOBP_Inflammatory Response pathway. Enrichment of the Inflammatory response is seen in the MEFS treated with the nephritic RM-MEF CM compared to the prenephritic (Young) RM-MEF CM. D. 18-hour scratch wound analysis of MEFs treated with conditioned media from the respective cocultures. Three biological replicates for each condition performed in duplicates. *p < 0.05 between MEFs treated with prenephritic and nephritic RM-MEF coculture CM.
To cite this abstract in AMA style:
Raparia C, Hoover P, Arazi A, Hacohen N, Davidson A. Resident Macrophages Localize Near Fibroblasts and Drive Reprogramming in Lupus Nephritis Through Direct and Soluble Signaling [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/resident-macrophages-localize-near-fibroblasts-and-drive-reprogramming-in-lupus-nephritis-through-direct-and-soluble-signaling/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/resident-macrophages-localize-near-fibroblasts-and-drive-reprogramming-in-lupus-nephritis-through-direct-and-soluble-signaling/
