Session Information
Session Type: Abstract Session
Session Time: 11:30AM-11:45AM
Background/Purpose: The goals of treatment for idiopathic inflammatory myopathy (IIM) are to eliminate or reduce inflammation, restore muscle performance, reduce morbidity, and improve a patient’s quality of life. Therapies providing durable meaningful clinical responses without requiring chronic administration are lacking. Rese-cel (formerly CABA-201), is a fully human, autologous 4-1BB CD19-CAR T cell therapy, designed to deeply and transiently deplete CD19 positive cells following a weight-based, single infusion with the potential to enable an “immune system reset” with durable responses. RESET-MyositisTM (NCT06154252) is an ongoing Phase 1/2 trial evaluating rese-cel in 3 independent cohorts of adults with dermatomyositis [DM], antisynthetase syndrome [ASyS] and immune mediated necrotizing myopathy [IMNM].
Methods: Patients were 18 to 75 years with refractory IIM per 2017 ACR/EULAR classification criteria, with myositis specific autoantibody (MSA), manual muscle test ≤142, at least moderate disease activity on 2 or more core set measures (CSMs). A weight-based infusion of 1×106 CAR T cells/kg was given following preconditioning with fludarabine and cyclophosphamide. All non-glucocorticoid (GC) IM agents were discontinued by Day –5. Adverse events, use of IM medications and efficacy measurements to calculate the TIS were collected. CAR T cells and B cells were profiled in peripheral blood pre- and post-infusion by digital PCR and flow cytometry, respectively. Serum cytokines were measured using the mesoscale discovery platform.
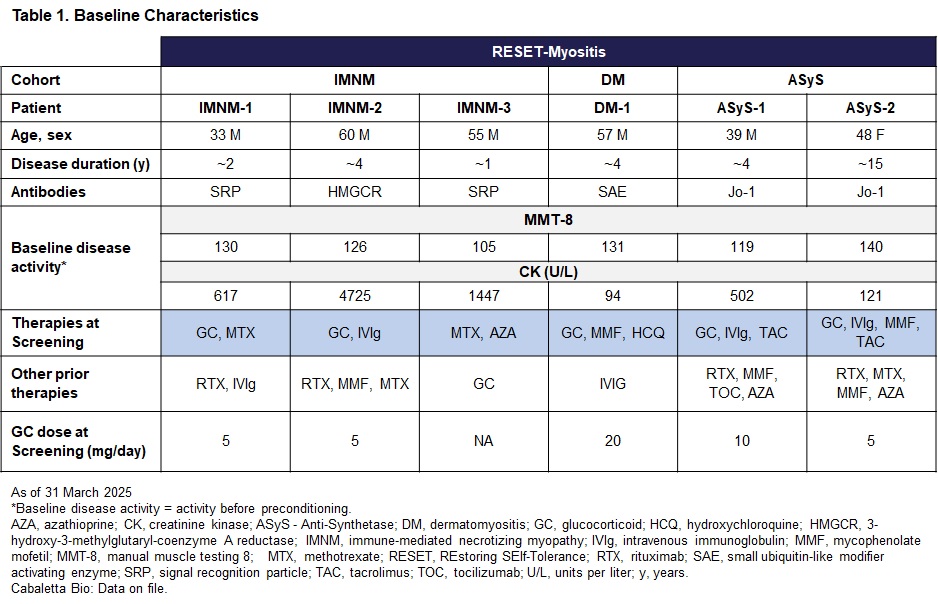
Results: As of 31 March 2025, 6 patients have been infused with rese-cel and have at least 1 month of follow-up (Table 1). Additional patients were recently infused and are in early follow-up.Rese-cel was well-tolerated in all 6 patients with Grade 1 cytokine release syndrome (CRS) in 3 of 6 patients, and no Grade 2 or higher CRS, dose-limiting toxicity or immune effector cell-associated neurotoxicity syndrome (ICANS). IMNM-2 with multiple risk factors, experienced a pulmonary embolism (PE) on Day 38, which was assessed as unrelated to rese-cel. Five out of 6 patients achieved a drug-free clinical response on TIS (Table 2).Rese-cel expanded rapidly in all patients and peaked between days 8 and 14 after infusion. Serum IFNγ levels increased post-infusion and peaked prior to rese-cel peak expansion. Rese-cel contracted quickly and was undetectable peripherally within the first 8 weeks in most patients. B cells (CD19+CD20+) were rapidly reduced to undetectable levels by 14 days and through the first month post rese-cel infusion in all patients. B cell reconstitution occurred as early as 8 weeks after infusion in 4 patients and 12 weeks in 1 patient. Reemergent B cells had predominantly transitional naïve phenotype (CD19+CD20+CD38++CD24++).
Conclusion: Data from IIM patients infused with rese-cel show early drug-free clinical responses in most patients and acceptable safety in the setting of rese-cel expansion and peripheral B cell depletion. DM & ASyS patients achieved a higher threshold of response than IMNM which is not unexpected. These initial data suggest the potential for rese-cel to reset the immune system, allowing patients to achieve meaningful drug-free clinical responses.
To cite this abstract in AMA style:
Wilfong E, Mozaffar T, Naddaf E, Chahin N, Lu H, Bauer Ventura I, Little C, diCasoli C, Miller C, Volkov J, Nunez D, Furmanak T, Stadanlick J, Ishikawa L, Vorndran Z, Ellis A, Williams J, Flanagan S, Lam Q, Hadi-Nezhad F, Tummala R, Basu S, Chang D. RESET-Myositis: Clinical Trial Evaluating Rese-cel (Resecabtagene Autoleucel), A Fully Human, Autologous 4-1BB CD19-CAR T Cell Therapy in Idiopathic Inflammatory Myopathies [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/reset-myositis-clinical-trial-evaluating-rese-cel-resecabtagene-autoleucel-a-fully-human-autologous-4-1bb-cd19-car-t-cell-therapy-in-idiopathic-inflammatory-myopathies/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/reset-myositis-clinical-trial-evaluating-rese-cel-resecabtagene-autoleucel-a-fully-human-autologous-4-1bb-cd19-car-t-cell-therapy-in-idiopathic-inflammatory-myopathies/

.jpg)