Session Information
Date: Sunday, November 12, 2023
Title: (0673–0690) Vasculitis – ANCA-Associated Poster I: Treatment Outcomes
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Although respiratory tract involvement in ANCA-associated vasculitis (AAV) is frequent and associated with increased mortality, studies focusing on diffuse alveolar hemorrhage (DAH) in AAV are uncommon. DAH can progress rapidly and is often life-threatening. The 330-patient ADVOCATE trial was an active-controlled, randomized, Phase 3 study that compared avacopan to a prednisone-tapering regimen and enrolled 12 patients with DAH.
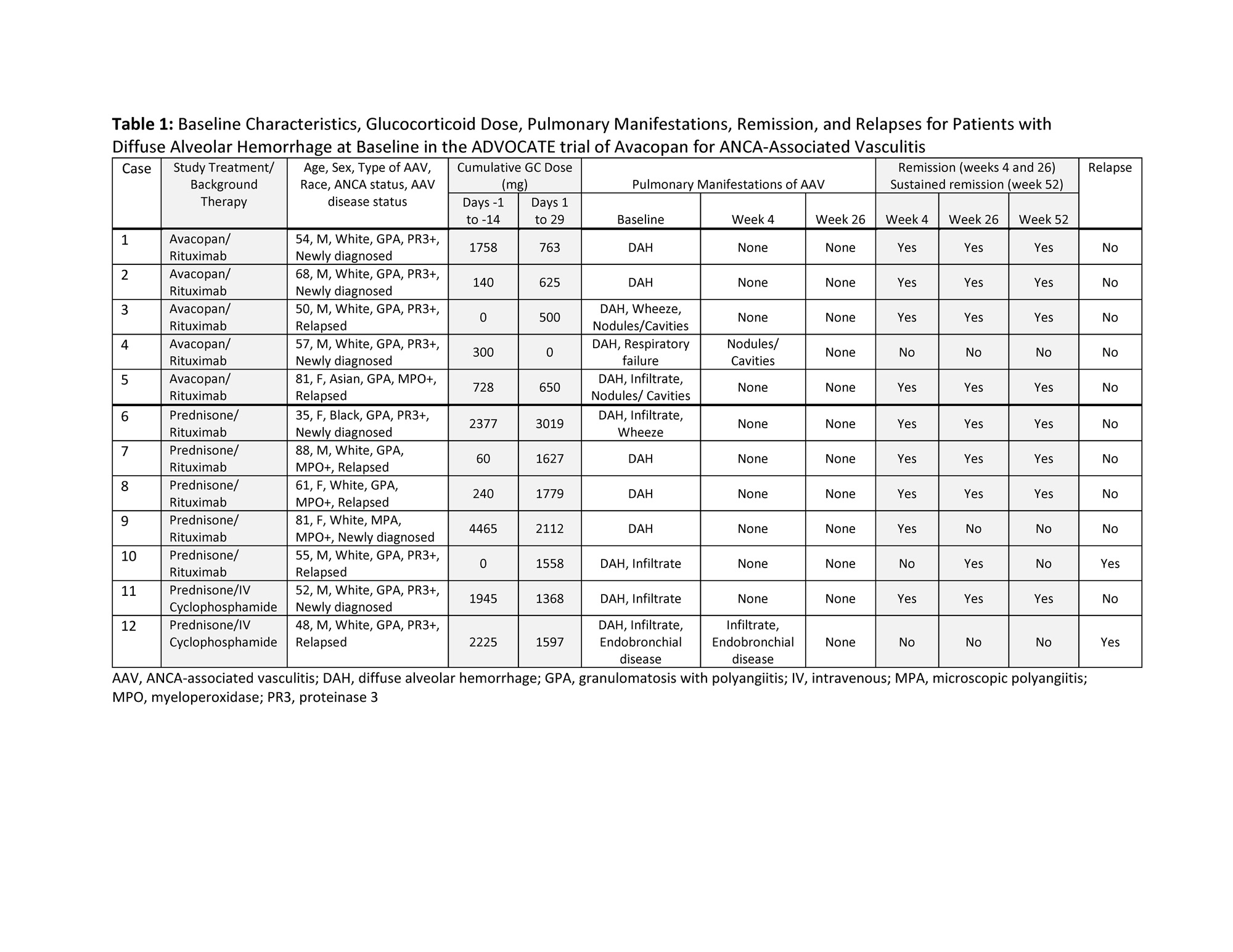
Methods: This post hoc analysis focuses on outcomes of the 12 patients with DAH at baseline on the basis of BVAS. Patients requiring invasive pulmonary ventilation support at screening were excluded from enrollment. Remission at Week 4 was defined as BVAS=0. Remission at Week 26 was defined as BVAS=0 and no glucocorticoid (GC) use in the previous 4 weeks. Sustained remission at Week 52 was defined as BVAS=0 and no GC use in the previous 4 weeks, remission at Week 26, and no relapse between Week 26 and Week 52. Relapse was defined as a return of vasculitis activity on BVAS with ≥1 major item, ≥3 minor items, or 1 or 2 minor items for at least two consecutive trial visits.
Results: Five patients in the avacopan group and 7 in the prednisone taper group had DAH at baseline. Of the 12 patients, 11 (92%) had granulomatosis with polyangiitis, 10 (83%) received rituximab background therapy, 8 (67%) were male, 8 (67%) had PR3-ANCA, and 6 (50%) were newly diagnosed (Table 1). BVAS was 19.5±5.4 (mean±SD) at baseline. The total dose of GCs (median / mean) from all sources for the avacopan and prednisone taper groups, respectively, were 300 / 585 mg vs 1945 / 1616 mg during the screening period (day -14 to -1) and 625 / 508 mg vs 1627 / 1866 mg during day 1 to 29. DAH was no longer active by Week 4 for all patients (Table 1). Remission rates in the avacopan and prednisone taper groups, respectively, were 80.0% (4/5) and 71.4% (5/7) at Week 4, 80.0% (4/5) and 71.4% (5/7) at Week 26, and 80.0% (4/5) and 57.1% (4/7) at Week 52. No patients in the avacopan group and 2 in the prednisone taper group relapsed during the treatment period. One patient in the avacopan group (case 3) was hospitalized twice for pneumonia. Two in the prednisone taper group had hospitalizations, twice for one patient (case 9) due to hepatocellular injury andherpes keratitis and once for the other patient (case 12) for worsening of AAV. Prednisone taper treatment was not completed in case 9 due to a serious adverse event of lymphopenia (day 33). None of the patients required mechanical ventilation during the study.
Conclusion: In the ADVOCATE trial of patients with AAV, the outcomes of patients with DAH were similar for those treated with avacopan versus with a prednisone taper. None of the patients with DAH in the avacopan group progressed to respiratory failure, despite receiving a minimal dose of GCs. In this report on a small number of patients, overall remission rates were higher in the avacopan group than the prednisone taper group at Weeks 4, 26, and 52, although all pulmonary manifestations of AAV were resolved in all patients by Week 26. These data provide support for the treatment of DAH in AAV using avacopan with a lower dose of GCs.
To cite this abstract in AMA style:
Specks U, Jayne D, Merkel P. Report on Twelve Patients with Diffuse Alveolar Hemorrhage in the Phase 3 Trial of Avacopan for the Treatment of ANCA-Associated Vasculitis [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/report-on-twelve-patients-with-diffuse-alveolar-hemorrhage-in-the-phase-3-trial-of-avacopan-for-the-treatment-of-anca-associated-vasculitis/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/report-on-twelve-patients-with-diffuse-alveolar-hemorrhage-in-the-phase-3-trial-of-avacopan-for-the-treatment-of-anca-associated-vasculitis/