Session Information
Session Type: Poster Session B
Session Time: 9:00AM-10:30AM
Background/Purpose: Immune checkpoint inhibitors (ICI) have shown great promise in the treatment of different malignancies. The use of ICIs has been associated with toxicities that are referred to as immune related adverse events (irAE). Rheumatologic irAEs, such as inflammatory arthritis and inflammatory myositis, have been described. Remitting seronegative symmetrical synovitis with pitting edema (RS3PE) is a condition characterized by pitting edema in the distal extremities in addition to findings of synovitis on exam with negative serology for RF. RS3PE has been described as a paraneoplastic syndrome in association with solid organ tumors and hematologic malignancies. To date, 9 case reports of RS3PE-like syndrome (RS3PE-LS) in association with ICI have been reported in the literature. In one case, the patient received both ICI and a tyrosine kinase inhibitor, while in another case, the patient received ICI and a poly (ADP-ribose) polymerase inhibitor. We present a series of 10 patients, who developed RS3PE-LS after starting on ICI.
Methods: The Canadian Research Group of Rheumatology in Immuno-Oncology (CanRIO) database, which includes a large prospective cohort of oncology patients on ICI therapy across Canada, was reviewed for all cases of RS3PE-LS. Data including the type of malignancy, ICI therapy, clinical manifestations, time of onset and response to treatment were assessed.
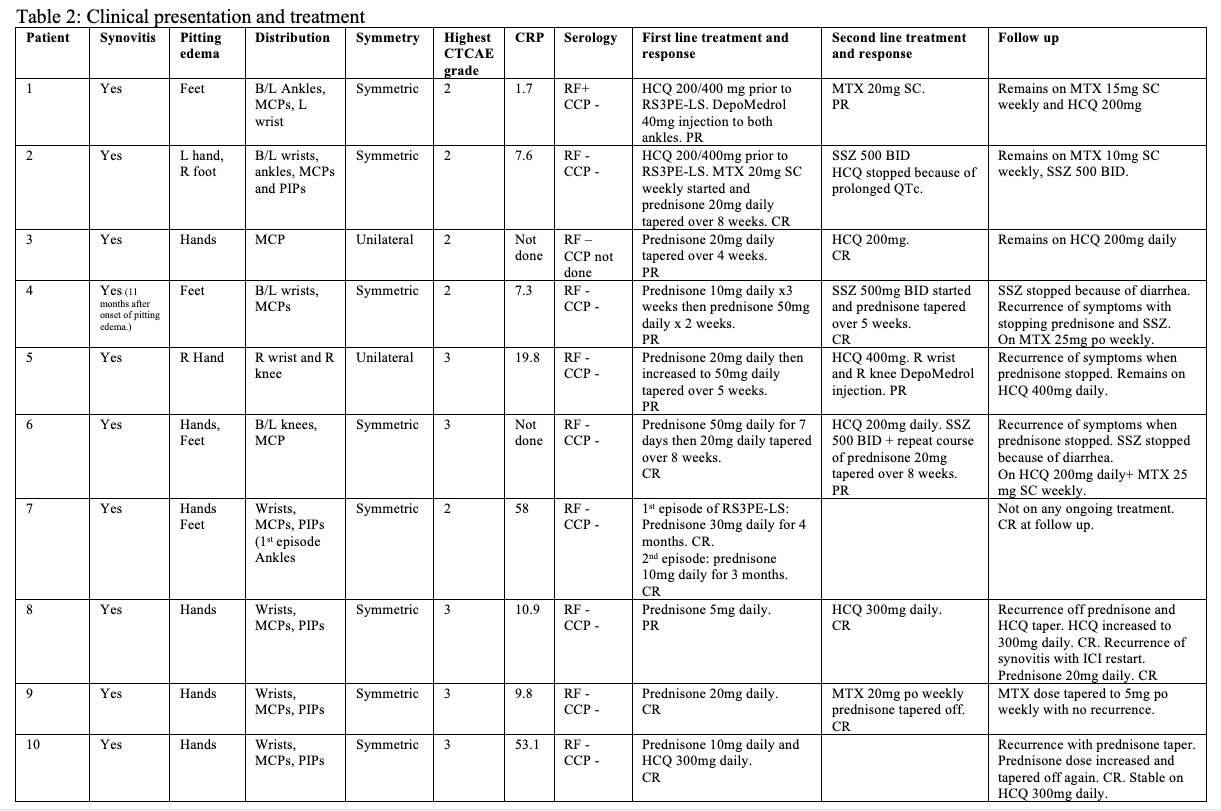
Results: Ten patients, who received ICI therapy for malignancy and presented with RS3PE-LS were identified (Table 1). The mean age of the patients was 72.9 years and 6/10 were women. The median time from start of ICI therapy to presentation with RS3PE-LS was 26 weeks, while the median time from the diagnosis of malignancy to onset of RS3PE-LS was 63 weeks. None of the patients had heart failure, liver cirrhosis or nephrotic syndrome. Seven out of 10 patients had stable cancer on imaging prior to onset of RS3PE-LS, while 2 had partial response and 1 was in complete remission. Three patients had completed their course of ICI therapy prior to onset of RS3PE-LS.
Pitting edema and synovitis was present in all patients. Symmetric involvement of joints was observed in 8/10 patients. Nine patients had negative serology, while 1 patient had positive RF. Eight out of 10 patients received prednisone as first line therapy, with starting doses ranging from 5-50 mg/day. The remaining two were already on hydroxychloroquine (HCQ) for inflammatory arthritis prior to RS3PE-LS, of which one was treated with prednisone after the development of pitting edema and the other received intra-articular steroid injection into the ankles. Two patients were started on a conventional synthetic DMARD (cs-DMARD) at the time of prednisone initiation (1Methotrexate (MTX), 1 HCQ). Nine out of 10 patients required additional cs-DMARD for management of their RS3PE-LS. Seven patients were continued on ICI or re-started on ICI after it was held, of which 3 patients remained in remission on cs-DMARD, 3 flared on cs-DMARD and one flared off cs-DMARD or prednisone therapy.
Conclusion: RS3PE-LS is a potential complication of ICI therapy. RS3PE-LS can flare after re-initiation of ICI and occur after ICI discontinuation. Current management varies widely and optimal treatment remains to be determined.
*Based on Response Evaluation Criteria in Solid Tumors (RECIST)
To cite this abstract in AMA style:
Rouhi A, Jamal S, Hudson M, Pope J, Roberts J, Ladouceur A, Hewitt S, Ye C. Remitting Seronegative Symmetrical Synovitis with Pitting Edema (RS3PE)-like Syndrome After Immune Checkpoint Inhibition: A CanRIO Study [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/remitting-seronegative-symmetrical-synovitis-with-pitting-edema-rs3pe-like-syndrome-after-immune-checkpoint-inhibition-a-canrio-study/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/remitting-seronegative-symmetrical-synovitis-with-pitting-edema-rs3pe-like-syndrome-after-immune-checkpoint-inhibition-a-canrio-study/