Session Information
Session Type: Abstract Session
Session Time: 3:00PM-4:30PM
Background/Purpose: Health services studies increasingly rely on Electronic Health Record (EHR) data to capture medication use. However, the reliability of medication information derived from EHR data (which is based on medication reconciliation and electronic prescriptions) compared to data from insurance claims (which represents administration or dispensations), especially for high-cost drugs such as biologic DMARDs, is unknown. We used data from the ACR’s Rheumatology Informatics System for Effectiveness (RISE) registry and linked Medicare claims to assess whether biologics documented in the EHR were also captured in insurance claims.
Methods: Data derived from RISE, a national, EHR-enabled registry that passively collects data on all patients seen by participating rheumatology practices, and Medicare data linked via social security numbers. Included beneficiaries were ≥ 65 years old, had ≥ 2 visits in RISE with RA ICD codes ≥ 30 days apart, and continuous enrollment in full-year Medicare Parts B and D in 2017-2018; patients enrolled in Medicare Advantage plans were excluded. We identified biologic users in RISE in 2018 (collected via medication reconciliation, electronic prescription orders, and procedure codes) and searched for corresponding Medicare claims in 2018 (outpatient, carrier, and Part D files). Medication records from both sources were identified using brand and generic names, CPT, and NDC codes. We reported the proportion of matched biologics, defined as instances where the same drugs documented in RISE were identified in Medicare claims, stratified patient dual eligibility for Medicare and Medicaid and whether medications were paid for primarily through Part B (facility or provider-administered) or Part D (pharmacy-dispensed).
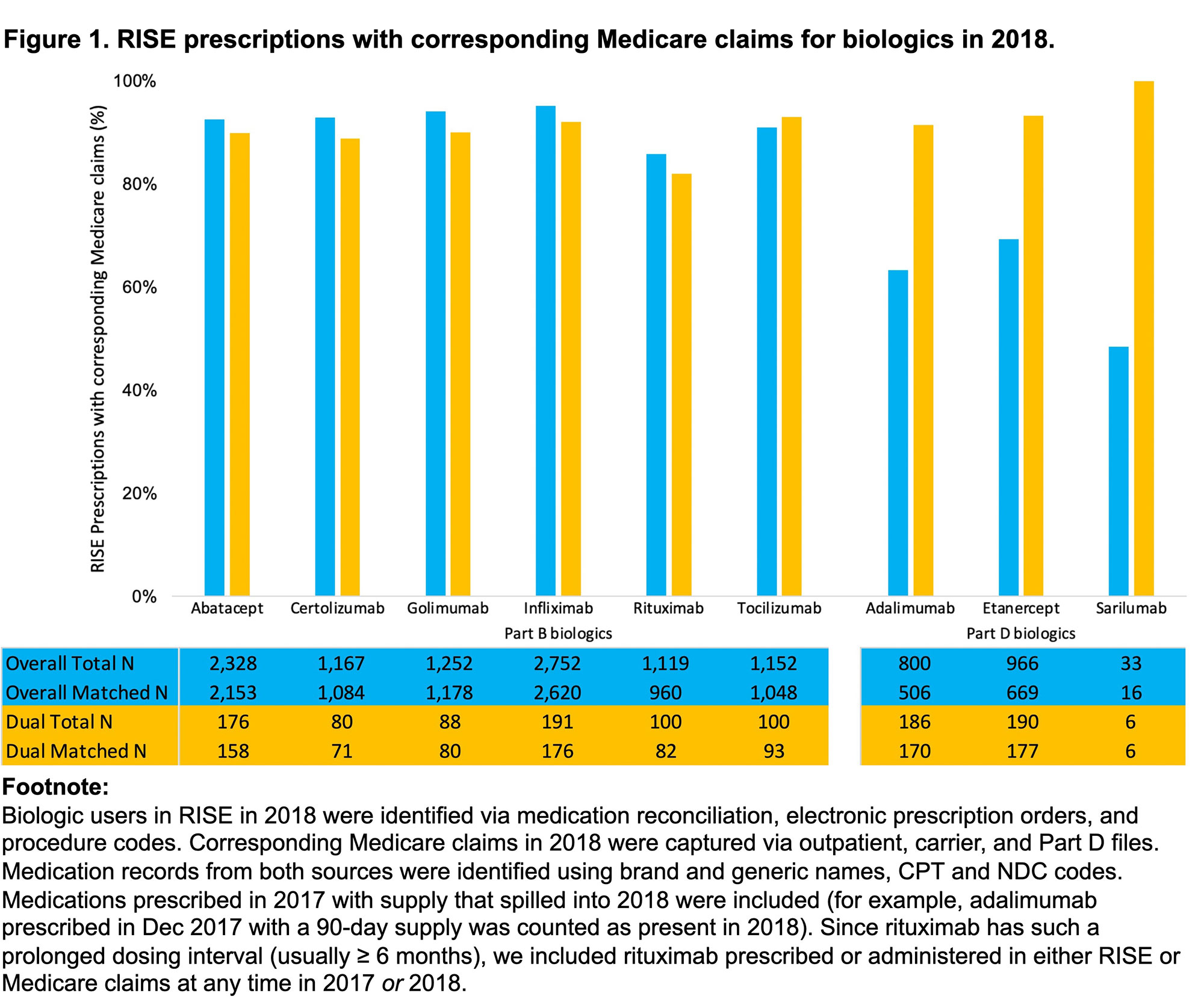
Results: 10,339 beneficiaries had 11,569 biologics recorded in RISE in 2018. Beneficiaries were 77.6% female, 85.5% non-Hispanic white, with mean (SD) age 74.3 (5.8); 9.8% had dual Medicare/Medicaid coverage (Table 1). The most commonly prescribed biologics were infliximab (26.6%), abatacept (22.5% – almost all intravenous (IV)) and golimumab (12.1% – also almost all IV). Overall, 88.5% (10,234/11,569) of prescriptions in RISE had a corresponding claim in Medicare. For biologics reimbursed primarily via Medicare Part B, concordance with Medicare claims were very high (85.6% – 95.2%). For biologics reimbursed primarily via Medicare Part D, concordance was lower (48.5% – 69.3%) (Figure 1). We observed higher concordance in both Part B and Part D for beneficiaries who were dual-eligible (82.0-100% across all biologics).
Conclusion: A large majority of biologics documented in RISE have corresponding Medicare claims, suggesting that misclassification of drug exposure is likely small in studies that rely on EHR data alone. Higher concordance for Part D biologics for dual-eligible beneficiaries are likely because low pharmacy drug costs facilitate adherence. Possible cost-related non-adherence or use of free drug samples by non-dual-eligible beneficiaries requires further exploration.
To cite this abstract in AMA style:
Baker R, Li J, Stovall R, Curtis J, Xie F, Yazdany J, Schmajuk G. Reliability of Medication Information Derived from EHR Data Compared to Insurance Claims: An Analysis of Biologic Medications in RISE and Medicare [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/reliability-of-medication-information-derived-from-ehr-data-compared-to-insurance-claims-an-analysis-of-biologic-medications-in-rise-and-medicare/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/reliability-of-medication-information-derived-from-ehr-data-compared-to-insurance-claims-an-analysis-of-biologic-medications-in-rise-and-medicare/