Session Information
Date: Friday, November 6, 2020
Title: RA – Diagnosis, Manifestations, & Outcomes II: The Heart of the Matter (0484–0488)
Session Type: Abstract Session
Session Time: 4:00PM-4:50PM
Background/Purpose: Paraoxonase 1 (PON1) is a high-density lipoprotein (HDL)‑associated enzyme with paraoxonase, lactonase, and arylesterase activities. PON1 contributes to the antioxidant properties of HDL, and is being investigated for its atheroprotective properties.1 Patients (pts) with RA that are homozygous for the RR genotype of the Q192R gene polymorphism on PON1 (rs662) have increased paraoxonase activity, and a lower risk of carotid plaques, vs those with QQ or QR genotypes.2 Tofacitinib is an oral Janus kinase inhibitor for the treatment of RA. This post hoc analysis investigated the relationship between PON1 genotype/activity and risk of major adverse cardiovascular events (MACE) in the tofacitinib RA clinical program.
Methods: Data were pooled from pts enrolled in nine Phase 2/3 studies of tofacitinib in RA. Enzyme activities in pt plasma samples were measured at individual study baseline (BL) and at follow‑up visits using three substrates: paraoxon (paraoxonase activity), dihydrocoumarin (lactonase activity), and phenylacetate (arylesterase activity). The effect of the PON1 Q192R genotype (QQ, QR, or RR) on BL paraoxonase/lactonase/arylesterase activity was determined using linear regression for each study with age and sex as covariates, and then fixed‑effect meta-analysis assessed effects across studies. The risk of MACE by enzyme activity was determined using Cox proportional hazards regression stratified by clinical studies. Univariate regression against BL enzyme activity and other risk factors, as well as both minimally and fully adjusted multivariable regressions against time-varying enzyme activity, are presented.
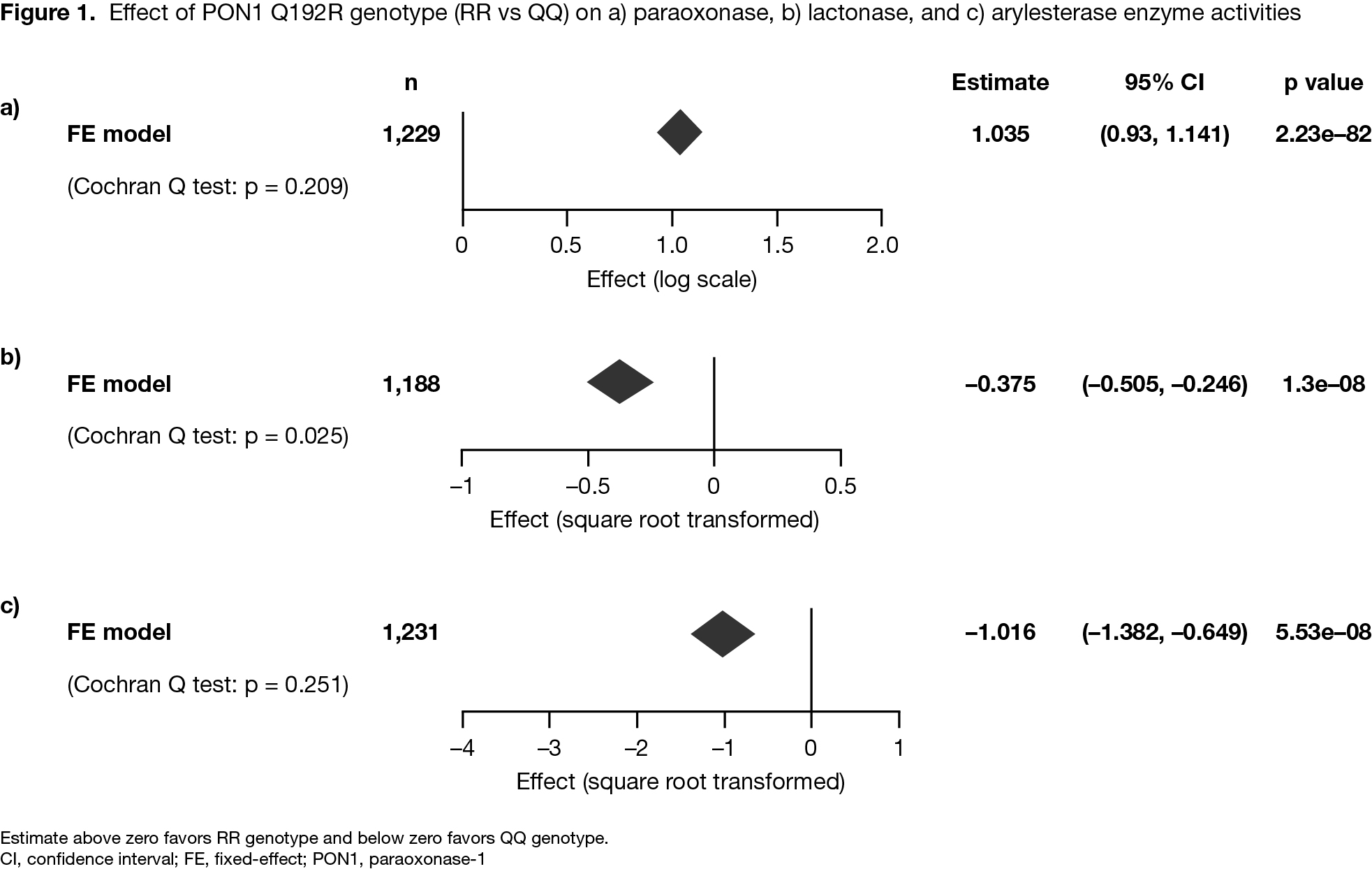
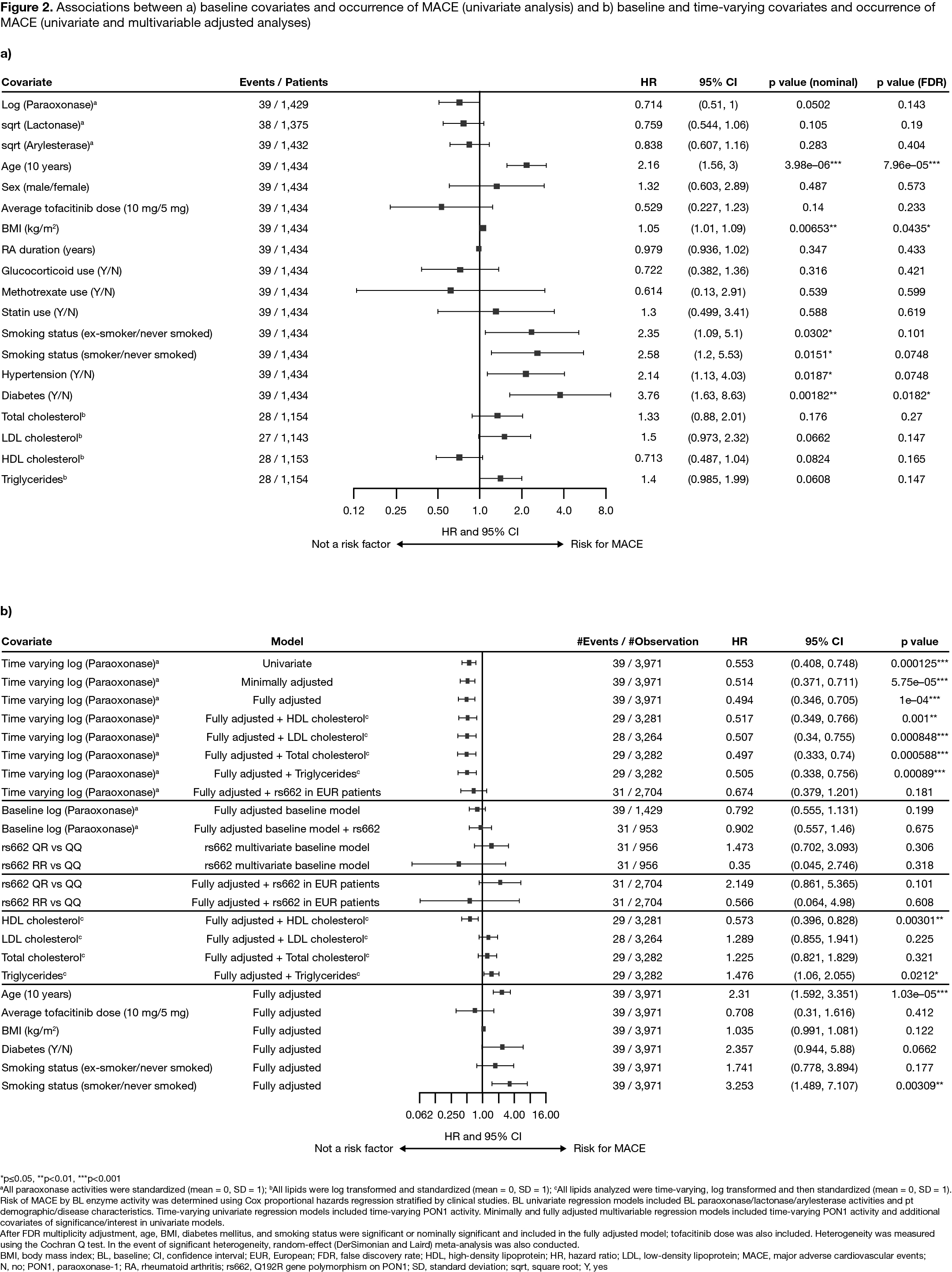
Results: The analysis included 1,969 pts with RA who received ≥ 1 dose of tofacitinib and had PON1 activity measures available at BL; 39 pts had ≥ 1 MACE event. Compared with the QQ genotype, the RR genotype had a highly significant positive association with BL paraoxonase activity, and a highly significant negative association with BL lactonase and arylesterase activity (Figure 1). A univariate analysis identified several BL covariates significantly associated with risk of MACE (Figure 2a). Time-varying models found a highly significant association of increased paraoxonase activity over time with lower risk of future MACE, even after controlling for low-density lipoprotein or HDL cholesterol levels, and other traditional cardiovascular (CV) risk factors identified in univariate analysis (Figure 2b), with similar findings for lactonase and arylesterase (data not shown).
Conclusion: Higher activity of the HDL-associated protein, PON1, over time was associated with a significantly reduced risk of future MACE in pts with RA receiving tofacitinib, after controlling for traditional CV risk factors and cholesterol levels. Further investigation of PON1 as a novel functional lipid biomarker to assess CV risk in RA pts is warranted.
- Mackness M, Mackness B. Gene 2015; 567: 12-21.
- Charles-Schoeman C et al. Arthritis Rheum 2013; 65: 2765-2772.
Acknowledgments: Study sponsored by Pfizer Inc. Medical writing support was provided by Jennifer Higginson, PhD, CMC Connect, and funded by Pfizer Inc.
To cite this abstract in AMA style:
Charles-Schoeman C, Hyde C, Guan S, Parikh N, Wang J, Shahbazian A, Stockert L, Andrews J. Relationship Between Paraoxonase-1 Genotype, Activity, and Major Adverse Cardiovascular Events in Patients with Rheumatoid Arthritis Receiving Tofacitinib [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/relationship-between-paraoxonase-1-genotype-activity-and-major-adverse-cardiovascular-events-in-patients-with-rheumatoid-arthritis-receiving-tofacitinib/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/relationship-between-paraoxonase-1-genotype-activity-and-major-adverse-cardiovascular-events-in-patients-with-rheumatoid-arthritis-receiving-tofacitinib/