Session Information
Date: Monday, November 13, 2023
Title: (1155–1182) Muscle Biology, Myositis & Myopathies – Basic & Clinical Science Poster II
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Our aim was to explore whether maintenance of remission in patients with idiopathic inflammatory myopathy (IIM) depends on glucocorticoids (GCs) after achieving remission. Therefor we aimed to evaluate long-term outcomes after GC-free remissions in patients with IIM and to determine factors associated with relapses. Secondly since the IMACS criteria for remission and relapse in IIM had not been fully validated [1], we examined their compatibility with statements of physicians.
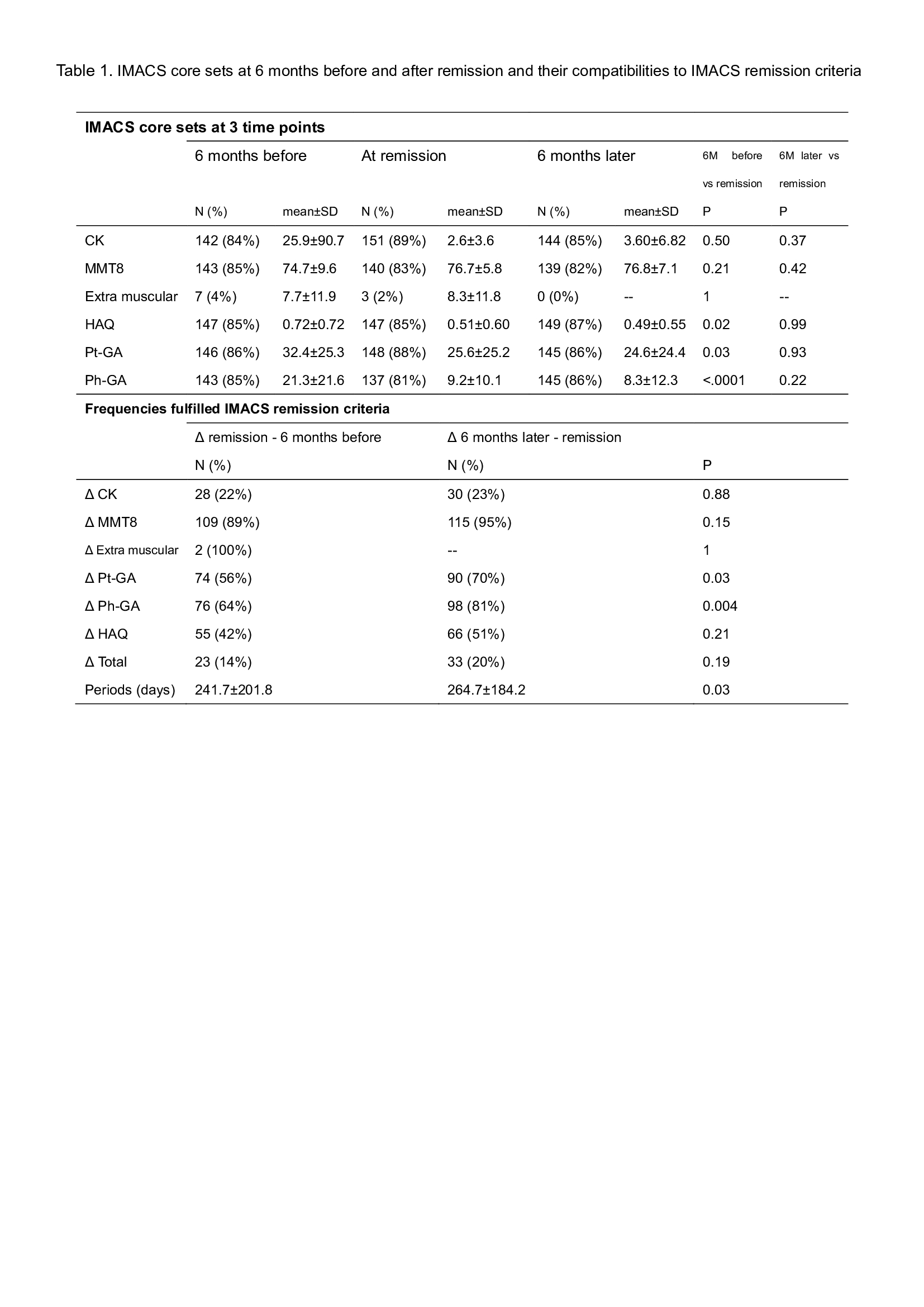
Methods: Data from prospectively followed patients with IIM in the Swedish Rheumatology Quality Register (SRQ) fulfilling the EULAR/ACR classification criteria were extracted and analyzed. For compatibilities of the definition of IMACS-remission, we investigated whether changes in IMACS core sets (CK, MMT8, extramuscular disease activity, patient-/physician-global assessment score (Pt-GA/Ph-GA), and health assessment questionnaire (HAQ)) were within 15% between 6 months before and after the physician’s statements of remission/inactive [1].
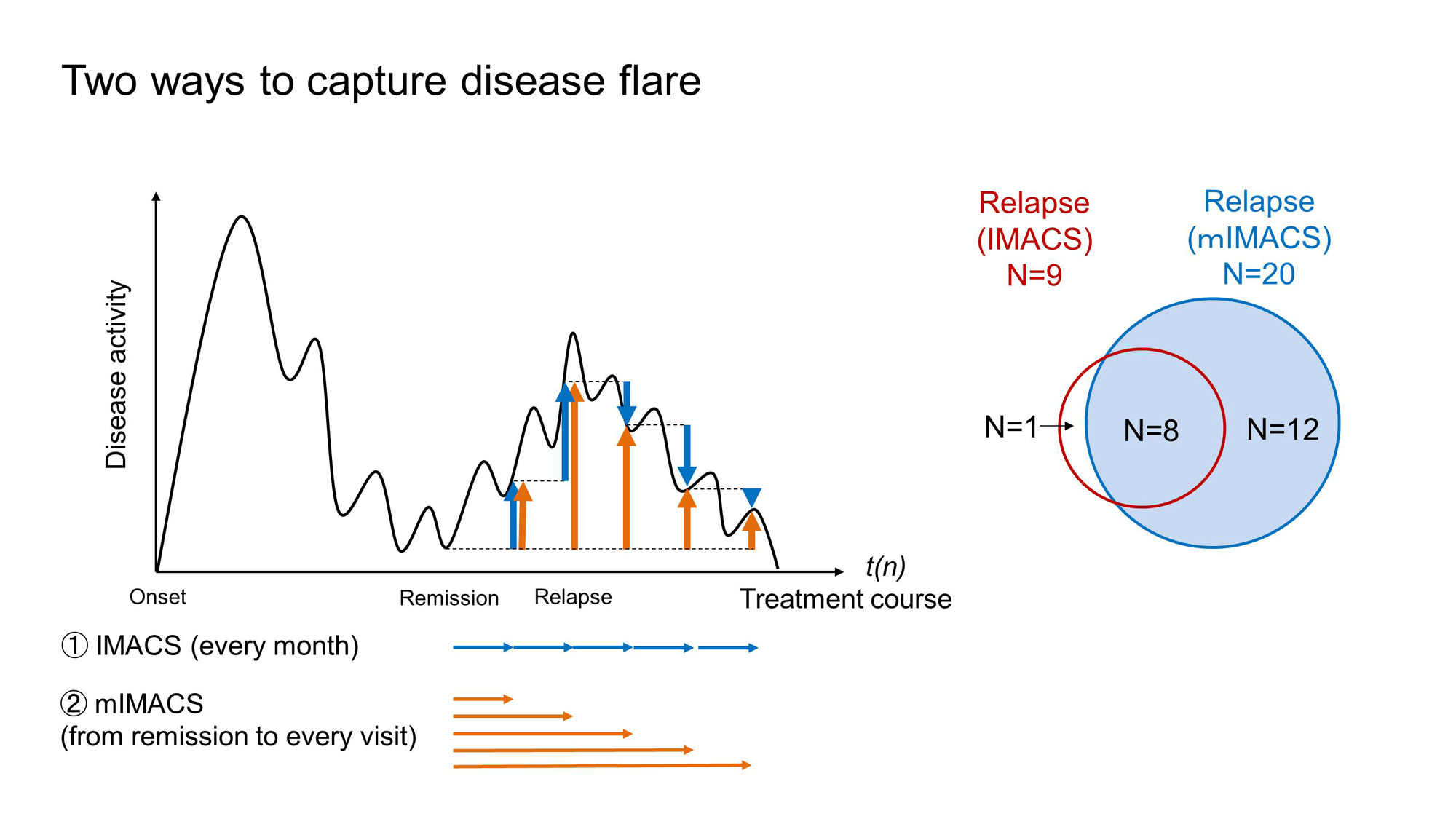
Relapse was defined as either fulfilling IMACS criteria for relapse (within 20–30% changes in a month) [1] or administration of GC/immunosuppression (IS), which were validated against IMACS relapse criteria. Furthermore, to increase the sensitivity of relapse, a modified IMACS (mIMACS) criteria for relapse was defined as not only changes every month but also changes from remission to a visit (Fig. 1). Relapses were examined for up to 14 years, and factors affecting relapses were also explored using logistic regression analyses.
Results: Of 697 patients with IIM, 192 patients were excluded because of lacking outcome data, 169 patients were identified with GC-free remission. Their IMACS core sets were not worsened over 6 months after the physician’s statement of remission/inactive. Variables that fulfilled IMACS-remission over 6 months before and after remission were CK (22%, 23%, respectively), MMT8 (89%, 95%), extra-muscular data (100%, no data), Pt-GA (56%, 70%), Ph-GA (64%, 81%), and HAQ (42%, 51%) (Table 1).
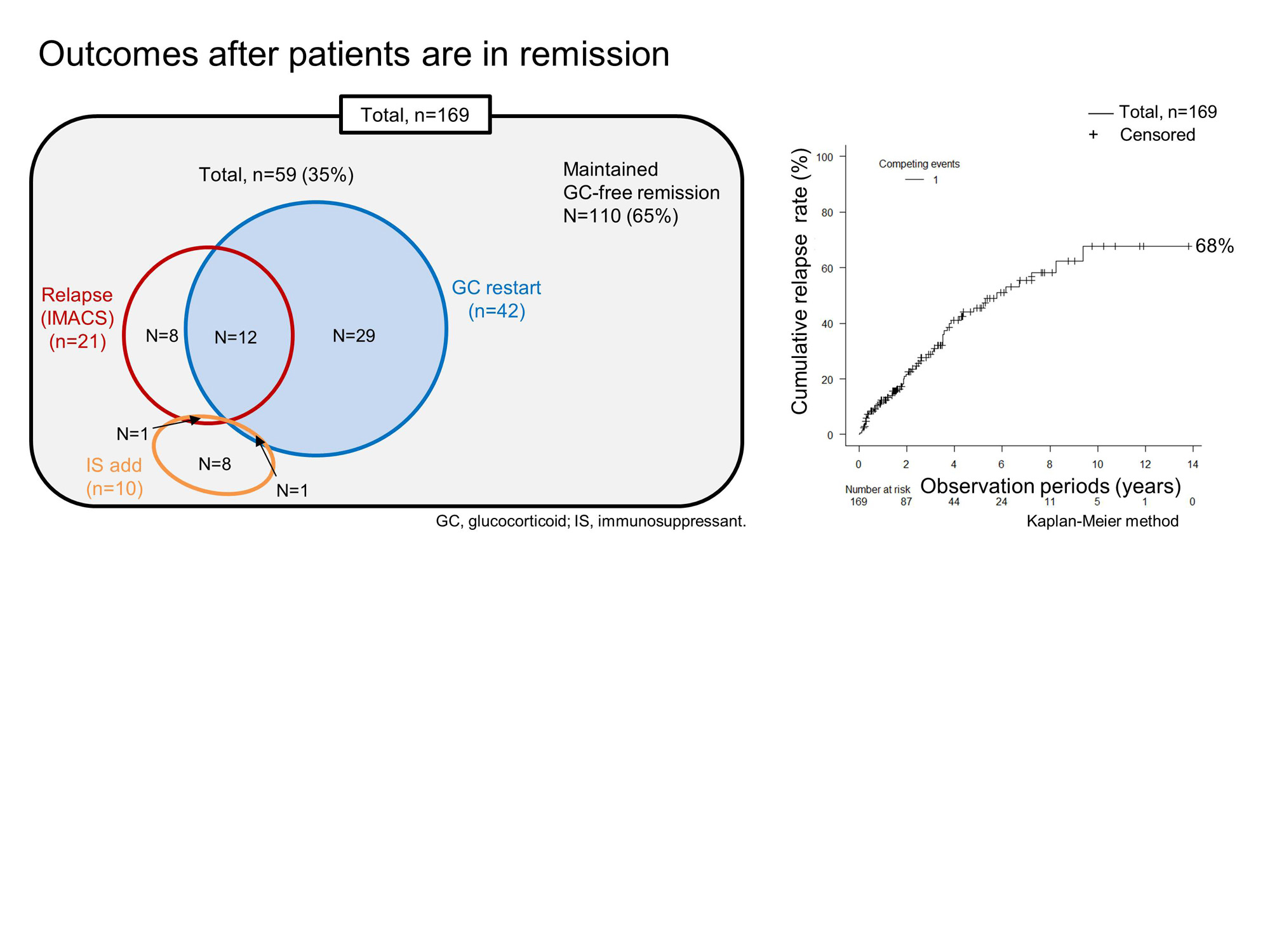
After GC-free remission, relapses were observed in 59 of the 169 patients, up to 9.4 years, with a cumulative relapse rate of 68% over 14 years for the Kaplan-Meier curve (Fig. 2). Twenty-one patients (36%) fulfilled IMACS criteria for relapse, including 9 patients with original IMACS criteria and 20 patients with mIMACS criteria (Fig. 1), in which IMACS core set was not significantly different. Relapses other than IMACS-relapse were defined as administration of GC/IS (n=38, Fig. 2). Characteristics in patients with IMACS-relapse were worsened CK, HAQ, Pt-GA, and Ph-GA. In contrast, Ph-GA and CRP worsened in patients with GC/IS defined relapse. Anti-Jo1 autoantibodies were shown as a risk factor for relapses by logistic regression analyses (odds ratio: 6.3, [95%CI: 1.11, 36.0]).
Conclusion: GC free remission using standard of care was achieved in 33% of patients with IIM. Cumulative relapse rate was recorded in 68% of these patients and occurred up to 9.4 years. Especially patients with anti-Jo1 autoantibodies are at risk to relapse. In addition, our real life data suggest that the criteria for remission and relapse in IMACS may need to be modified to fully capture disease flares.
References: 1) Arthritis Rheum. 2005;52(9):2607_15.
To cite this abstract in AMA style:
Tsuji H, Espinosa-Ortega F, Dastmalchi M, Lundberg I, Lodin K. Relapse Rate After Glucocorticoid-free Remission in Idiopathic Inflammatory Myopathies with Validation of the International Myositis Assessment & Clinical Studies Group (IMACS) Criteria for Remission and Relapse [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/relapse-rate-after-glucocorticoid-free-remission-in-idiopathic-inflammatory-myopathies-with-validation-of-the-international-myositis-assessment-clinical-studies-group-imacs-criteria-for-remission/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/relapse-rate-after-glucocorticoid-free-remission-in-idiopathic-inflammatory-myopathies-with-validation-of-the-international-myositis-assessment-clinical-studies-group-imacs-criteria-for-remission/