Session Information
Date: Tuesday, October 23, 2018
Title: Pediatric Rheumatology – Clinical Poster III: Juvenile Idiopathic Arthritis and Uveitis
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: In Canada, the pediatric indications of etanercept (ETN) are active ankylosing spondylitis (AS), plaque psoriasis (PsO) and moderate to severely active juvenile idiopathic arthritis (JIA; in those who have had an inadequate response to ≥1 DMARDs and are ≥4 years of age). A previous analysis of Canadian pediatric claims data showed a 78% yearly retention rate over Year 1 for ETN, which remained high over Years 2-6 (80-90% per year). However, changes in co-medication during ETN treatment in pediatric patients have rarely been evaluated in the real-world setting. Objective: To evaluate co-treatment utilization and ETN costs in Canadian pediatric patients initiating ETN therapy.
Methods: A retrospective study was conducted using longitudinal prescription drug claims data from the IQVIA Private Drug Plan, Ontario Public Drug Plan, and Quebec Public Drug Plan databases. Biologic-naïve pediatric patients (<18 years, with no biologic treatment in the preceding 12 months) were included if they initiated ETN during the selection period (Jan 2008-Jan 2016). Disease indications were inferred through patient drug history. Analyses of ETN doses and co-treatments were conducted in patients <17 years at index and with no missing data or drug histories indicative of conditions other than JIA, AS, or psoriatic arthritis. Weekly ETN dose was estimated for those who completed 12-month continuous ETN therapy (7 x [mg dispensed/days between claims]). Co-treatments were captured for the 6 months preceding and 12 months following index. Drug costs of ETN were estimated for those <18 years who initiated ETN therapy. Editorial support was provided by Jon Edwards, PhD, of Engage Scientific Solutions and was funded by Pfizer.
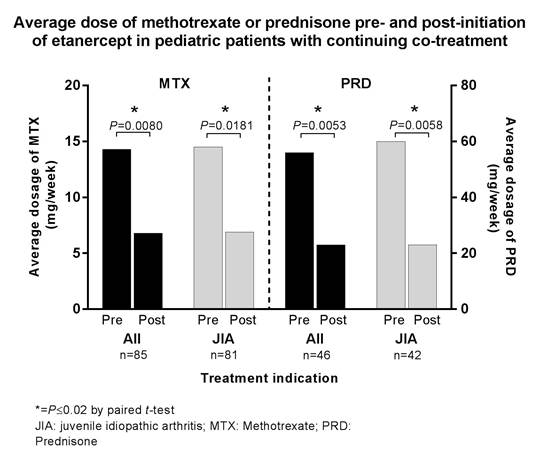
Results: The study identified 391 patients <18 years old who initiated ETN and had not received a biologic in the preceding 12 months. Of these, 330 provided data for the evaluation of ETN doses, co-treatments, and cost (67% female, 39% aged 10-14 years). Among the 316 patients who completed 12 months of continuous ETN therapy, the average weekly ETN dose was 31 mg (range 14-41 mg), but varied with age. Overall, 31% (n=103) used methotrexate (MTX) before initiating ETN, with 83% (n=85) continuing MTX through the first 12 months of ETN treatment; 28% (n=92) used prednisone (PRD) before initiating ETN, with 50% (n=46) continuing PRD during the first 12 months of ETN treatment. In patients continuing co-treatment, weekly dosages were significantly reduced (Figure). The average yearly cost of ETN was $13,671 (Canadian $ per year).
Conclusion: This evaluation of Canadian claims data demonstrated that nearly a third of pediatric patients initiating ETN were co-treated with MTX or PRD. Many patients discontinued their co-therapies, and weekly dosages of MTX or PRD were significantly lower within the first year of initiating ETN treatment for those who continued therapy with these agents.
Figure
To cite this abstract in AMA style:
Khraishi MMM, Millson B, Woolcott J, Marshall L, Jones H. Reduction in the Utilization of Prednisone and/or Methotrexate Following the Initiation of Etanercept in Pediatric Patients [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/reduction-in-the-utilization-of-prednisone-and-or-methotrexate-following-the-initiation-of-etanercept-in-pediatric-patients/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/reduction-in-the-utilization-of-prednisone-and-or-methotrexate-following-the-initiation-of-etanercept-in-pediatric-patients/