Session Information
Session Type: Poster Session C
Session Time: 1:00PM-3:00PM
Background/Purpose: The purpose with this study was to investigate whether the effects of a 4-week treatment with adalimumab (Humira™) could change previously reported aberrations in cerebral pain processing in patients suffering from rheumatoid arthritis (RA). Secondary aims were to identify pre-treatment markers in cerebral pain processing that potentially could predict the treatment response in RA patients.
Methods: RA patients were recruited through the rheumatology clinic at the Karolinska University Hospital. The study was registered at clinical trials.gov NCT01197144. Pre-treatment RA baseline task-based functional magnetic resonance imaging (fMRI) data and brain connectivity compared to healthy subjects have earlier been published (Flodin et al, Front in Hum Neurosci 2016; Sandstrom A, BBI 2019). To our knowledge, the current study comprises the largest fMRI sample size of RA patients (n = 27) to investigate the effects of TNF-α blockade on cerebral pain processing, as well as the first to use a randomized, double-blind, placebo-controlled design (Adalimumab (ADA) n = 14; Placebo (PBO) n = 13). Individually calibrated pressure pain stimuli were applied at the most inflamed interphalangeal joint, left hand, before and following treatment with ADA (40 mg s.c. every other week) or placebo injections. The fMRI analysis comprised a full factorial design to test interactions between [group x painful stimulus pre and post treatment] and its correlational relationship with relevant clinical parameters. All 2nd level analyses were adjusted for age and corrected for for multiple comparisons FWE < .05. The ADA treated group was divided into the lowest (n = 7, < /= 0.8 improvement in DAS28 ESR) and highest (n = 7, ( >0.8 improvement in DAS28 ESR) responding ADA treated RA patients. The 2nd level 2 sample t-test was performed on contrast ADA HIGH vs. ADA LOW [PainOnlyPre]*ESROnlyPre.
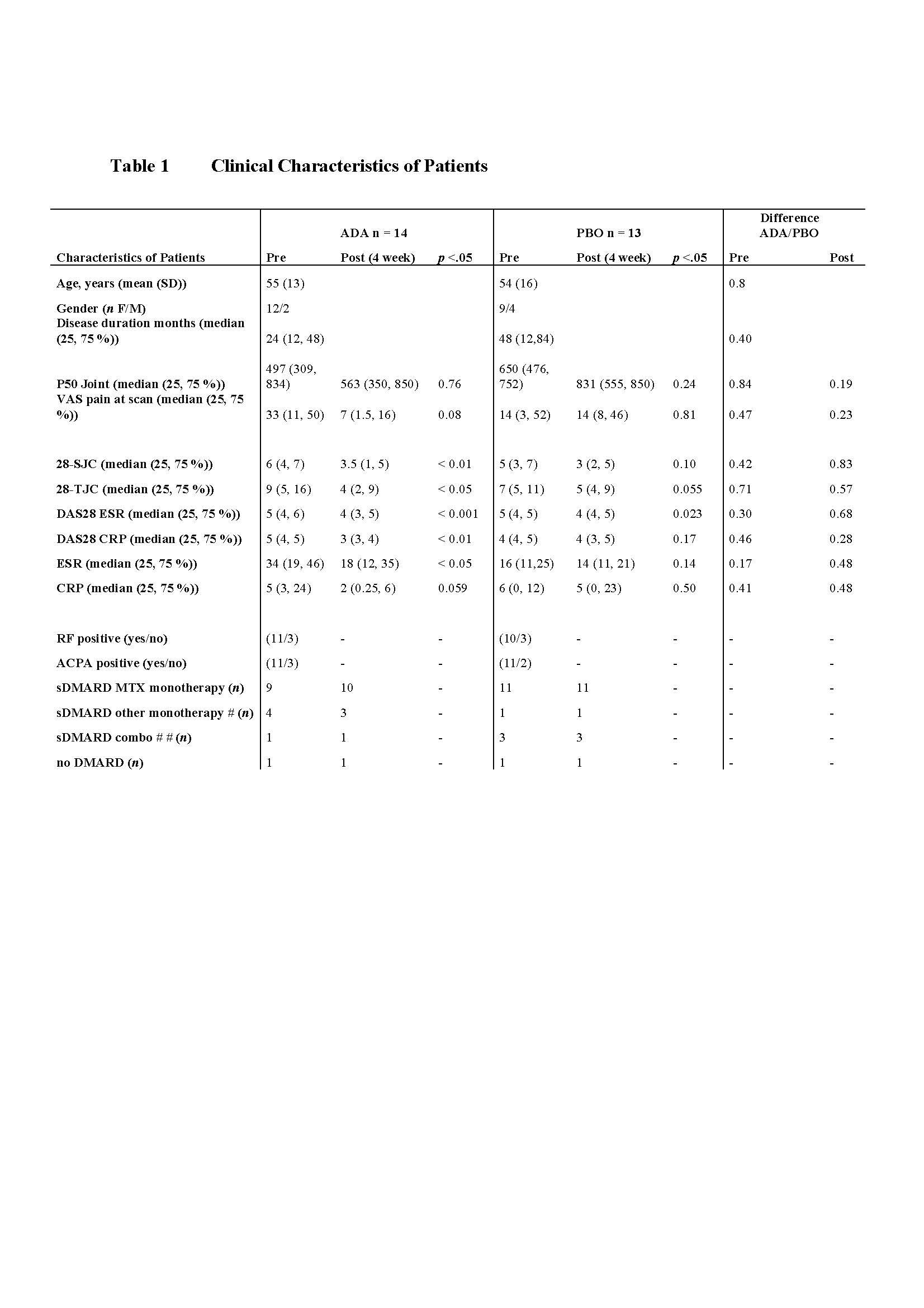
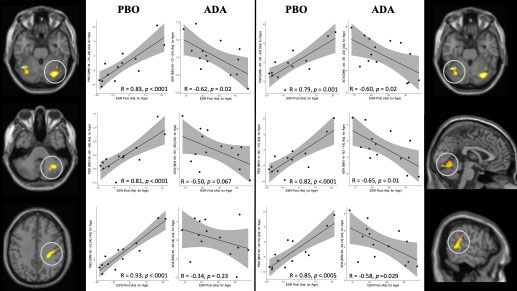
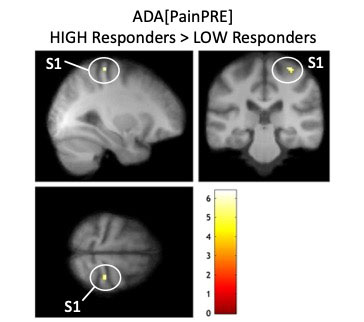
Results: The clinical results of 4 weeks ADA/PBO treatment are displayed in table 1. Concerning effects on cerebral pain processing, fMRI pre/post comparable analysis using erythrocyte sedimentation rate (ESR) as a covariate revealed an interaction between Group[ADA vs. PBO] x Pain[Pre vs. Post]*ESR[Pre vs. Post]. Following treatment, the ADA treated group exhibited decreased brain activation correlating with post-treatment ESR levels in cerebellum, right postcentral gyrus (S1) and right posterior midtemporal gyrus/inferior parietal lobe (IPL) compared to the PBO group. Conversely, the PBO group exhibited increased brain activation in left postcentral gyrus correlating with ESR levels, compared to the ADA group (figure 1).Within the ADA group, HIGH >LOW responders had statistically significantly more brain activation in R S1 prior to treatment initiation (baseline) in the right precentral gyrus ([MNI 30 -28 66]; t = 6.39; z-value = 4.05; PFWE = 0.033) (figure 2).
Conclusion: The current results support that TNF inhibitors do not only modulate peripheral inflammation, but also directly or indirectly influence cerebral pain processing. Moreover, our data implicate that the BOLD signal can be used as a potential marker for predicting clinical responses to therapeutic interventions in RA patients and other forms of inflammatory arthritis.
ADA = Adalimumab; RA = rheumatoid arthritis; ESR = erythrocyte sedimentation rate
Prior to treatment initiation, the highest ADA responders (n = 7) had significantly more pain-related brain activation within the primary somatosensory cortex (S1) compared to the lowest responding ADA treated RA patients (n = 7) (i.e. ADAHIGH>ADALOW[PainPRE]).
ADA = Adalimumab; RA = rheumatoid arthritis
To cite this abstract in AMA style:
Sandström A, Fransson P, Altawil R, Kosek E, Lampa J. Reduced Inflammation Following 4-weeks TNF-α Inhibitor Treatment Restore Aberrant Cerebral Pain Processing in Rheumatoid Arthritis Patients: A Randomized Double-Blind Placebo-Controlled fMRI Study [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/reduced-inflammation-following-4-weeks-tnf-%ce%b1-inhibitor-treatment-restore-aberrant-cerebral-pain-processing-in-rheumatoid-arthritis-patients-a-randomized-double-blind-placebo-controlled-fmri-stud/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/reduced-inflammation-following-4-weeks-tnf-%ce%b1-inhibitor-treatment-restore-aberrant-cerebral-pain-processing-in-rheumatoid-arthritis-patients-a-randomized-double-blind-placebo-controlled-fmri-stud/