Session Information
Session Type: Abstract Session
Session Time: 11:45AM-12:00PM
Background/Purpose: Dermatomyositis (DM) is a systemic autoimmune disease marked by muscle weakness and characteristic cutaneous manifestations. Dysregulation of the Type I interferon (IFN-I) pathway plays a crucial role in its pathogenesis. Anifrolumab (ANI), an anti-IFNAR1 monoclonal antibody approved for the treatment of lupus, is a potential treatment for IFN-I–mediated diseases. This study aims to assess the efficacy and safety of ANI in patients with refractory DM.
Methods: We performed a multicenter, multidisciplinary, retrospective study involving 24 DM patients treated with ANI under compassionate use. Data collected included demographics, clinical features, serology, prior treatments, and concomitant medications. Efficacy was measured using the Cutaneous Dermatomyositis Disease Area and Severity Index (CDASI) and the Manual Muscle Test 8 (MMT8). Safety was evaluated based on adverse events (AEs).
Results: Of the 24 patients, 83% were female, with a mean age of 58.1±21.1 years at DM diagnosis and 53.9±19.8 years at ANI initiation. The majority were Caucasian (79%). DM subtypes included classic (9), amyopathic (10), juvenile (3), and antisynthetase syndrome (2); 21% had a paraneoplastic origin. Common cutaneous manifestations included Gottron’s papules (100%), pruritus (92%), periorbital erythema (83%), periungual erythema (79%), alopecia (46%), and calcinosis (38%). Musculoskeletal involvement was observed in 58%, dysphagia in 42%, arthritis in 50%, and interstitial lung disease in 21%. Myositis-specific antibodies were detected in 19 patients: anti-TIF1γ (8), anti-MDA5 (3), anti-SAE (4), anti-ARS (3), anti-Mi-2 (1); 5 patients were seronegative. Anti-Ro52 was present in 9 (38%). Prior treatments included oral CS (100%), HCQ (88%), MTX (88%), AZA (25%), TAC (21%), MMF (67%), IVIG (79%), RTX (29%), TOFA (42%), BARI (8.3%), RUXO (4.1%), and others. ANI was initiated mainly for cutaneous refractoriness (91.6%) and musculoskeletal activity (8.4%). It was administered at 300 mg every 4 weeks, except in one juvenile DM patient, who received 150 mg every 4 weeks. Half of the patients received concomitant csDMARDs: MTX (4), MMF (4), TAC (2), AZA (1), and TAC + MMF (1). After a mean follow-up of 7.5±4.2 months, most patients showed marked cutaneous improvement (CDASI) and muscle stability (MMT8) (Figure 1). The impact of ANI on cutaneous manifestations over 12 months is shown in Figure 2. In two patients, the IVIG intervals were successfully spaced due to clinical improvement. One juvenile DM patient had a flare, with CK elevation (450 UI/L) and a positive Gowers’ sign after discontinuing MTX. AEs included impetigo (1), arthralgia/myalgia (1), urinary tract infections (2), and herpes zoster (1). No AEs led to ANI discontinuation, although one patient stopped treatment due to loss to follow-up.
Conclusion: Anifrolumab demonstrated early efficacy in dermatomyositis, with a favorable safety profile in refractory DM. This is the largest real-world series of DM patients treated with ANI to date, supporting its role in IFN-I–mediated dermatomyositis.
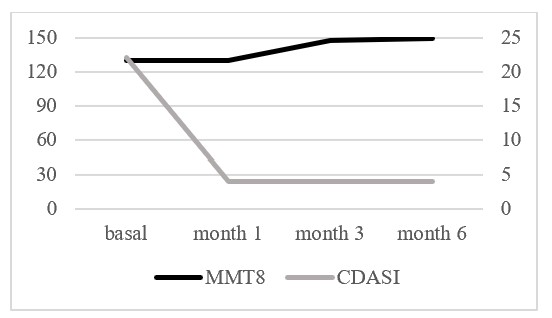 Figure 1. Evolution of CDASI and MMT8 after initiation of anifrolumab over a 6-month follow-up period.
Figure 1. Evolution of CDASI and MMT8 after initiation of anifrolumab over a 6-month follow-up period.
.jpg) Figure 2. Impact of anifrolumab on different cutaneous manifestations over 12 months of follow-up.
Figure 2. Impact of anifrolumab on different cutaneous manifestations over 12 months of follow-up.
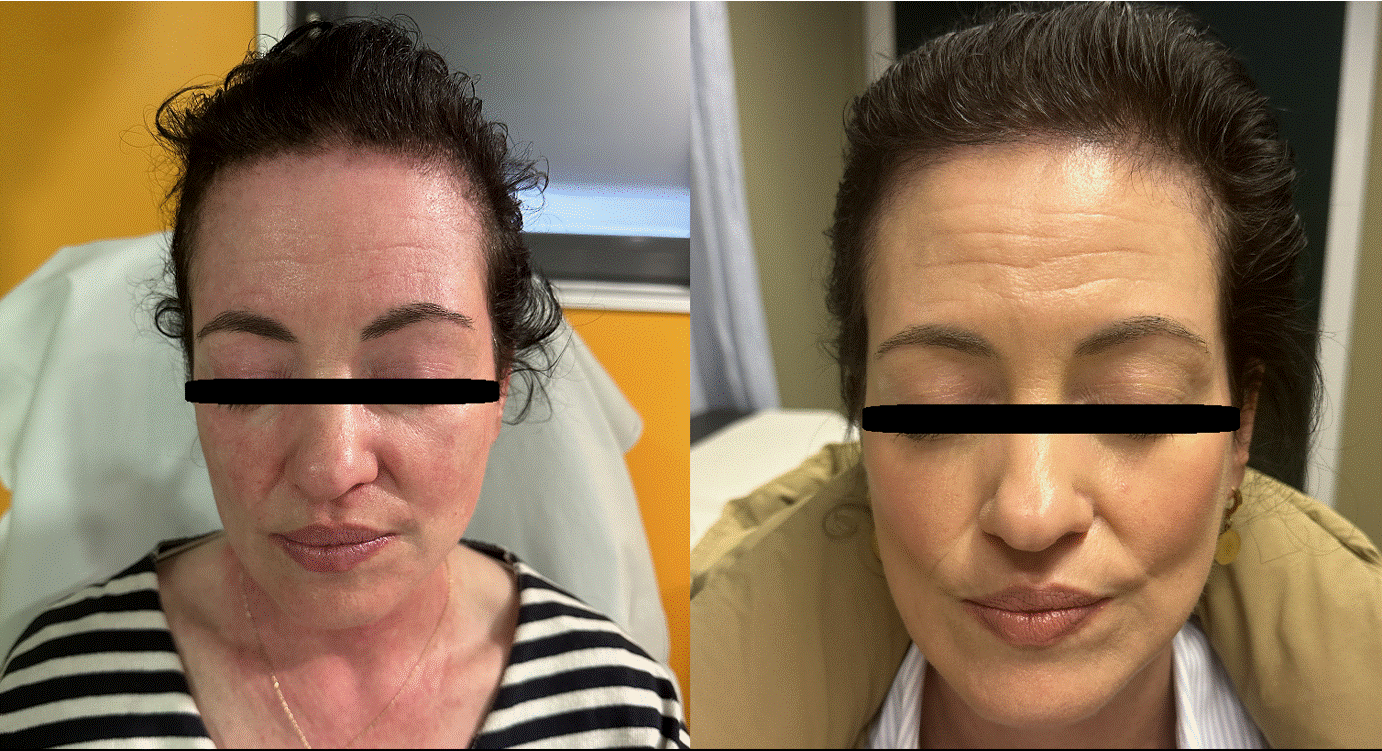 Figure 3. Significant improvement in facial and upper chest erythema observed following three doses of anifrolumab.
Figure 3. Significant improvement in facial and upper chest erythema observed following three doses of anifrolumab.
To cite this abstract in AMA style:
Retuerto Guerrero M, Moriano C, Pareja-Martínez A, Molina-Esteban N, Puig-Buendia J, Postigo-Llorente C, Lopez-Ceron Cofiño A, Martínez Barrio J, Vidal-Montal P, Sendagorta Cudos E, GONZALEZ ARRIBAS G, Calvo J, De Dios J, Blanco-Madrigal J, Vela Casasempere P, Bachiller J, Fito-Manteca C, Ordas calvo C, Martos Cabrera L, Sanchez Herrero A, Ortega-Valin L, Diez Alvarez E. Real-World Experience of Anifrolumab in 24 Patients with Refractory Dermatomyositis: A Multicenter Retrospective Study. [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/real-world-experience-of-anifrolumab-in-24-patients-with-refractory-dermatomyositis-a-multicenter-retrospective-study/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/real-world-experience-of-anifrolumab-in-24-patients-with-refractory-dermatomyositis-a-multicenter-retrospective-study/
