Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Upadacitinib (UPA), an oral JAK inhibitor, has demonstrated efficacy for the treatment of PsA in randomized controlled trials of patients with inadequate response or intolerance to biologic DMARDs1; however, there remains limited real-world data on the effectiveness of UPA. This study assessed real-world improvement in key clinical and patient-reported outcomes (PROs) among adult PsA patients initiating UPA after prior experience with a TNF inhibitor.
Methods: The OM1 PsA registry (January 2013-March 2024) was used to identify TNF inhibitor-experienced PsA patients who initiated UPA on or after December 14, 2021. The OM1 PsA Registry (OM1, Inc; Boston, MA) follows > 60k PsA patients in the US managed by rheumatologists longitudinally with clinical data and linked administrative claims. Inclusion criteria were defined as ≥ 18 years of age, ≥ 1 UPA claim (index date defined as the first UPA claim), ≥ 1 claim of TNF inhibitor before index date, ≥ 6 months of available data before index date (ie, baseline period), ≥ 1 baseline outcome measure, and ≥ 1 follow-up outcome measure (3- or 6-months post index). Mean changes from baseline were evaluated at both 3 and 6 months for the following clinical outcomes and PROs: RAPID3, TJC28, SJC28, pain by visual analog scale (VAS: 0-10), fatigue by numeric rating scale (NRS: 0-10), Multi-Dimensional Health Assessment Questionnaire (MDHAQ) Physician Global Assessment (PGA: 0-10), and MDHAQ Patient Global Assessment (PtGA: 0-10). For each outcome, paired t tests were performed to test the difference between the baseline and follow-up scores at 3- and 6-months post index.
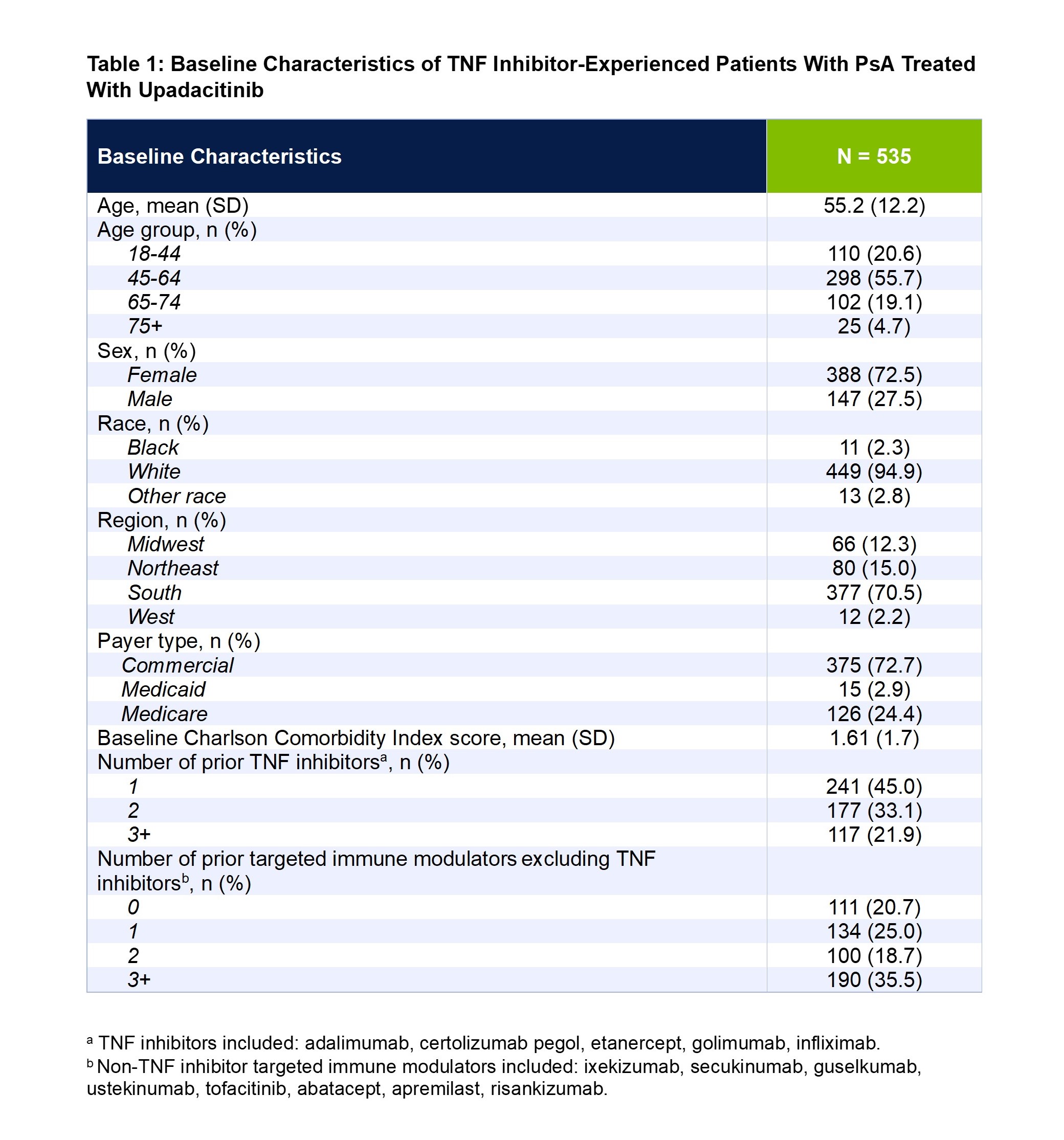
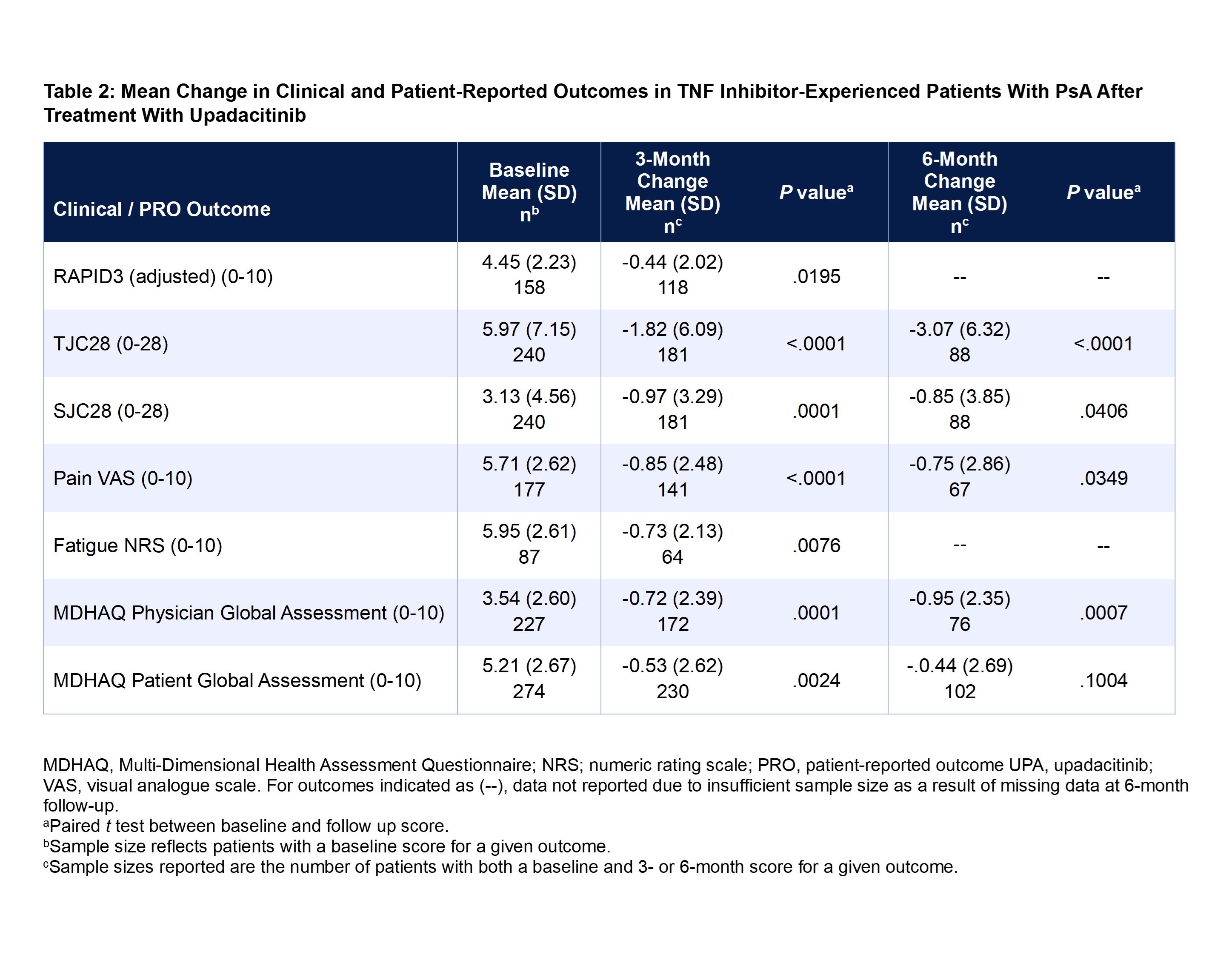
Results: A total of 535 TNF inhibitor-experienced patients with PsA met the inclusion criteria. Mean patient age was 55.2 years, 73% of patients were female, and 95% were White. Most patients were covered under commercial insurance (73%), followed by Medicare (24%), and Medicaid (3%) (Table 1). At baseline, overall mean ± SD scores were 4.45 ± 2.23 for RAPID3, 5.97 ± 7.15 for TJC28, 3.13 ± 4.56 for SJC28, 5.71 ± 2.62 for pain, 5.95 ± 2.61 for fatigue, 3.54 ± 2.60 for MDHAQ PGA, and 5.21 ± 2.67 for MDHAQ PtGA. At 3 months, significant improvements (all P < 0.05) were observed for all the clinical outcomes and PROs: RAPID3 (-0.44 ± 2.02), TJC28 (-1.82 ± 6.09), SJC28 (-0.97 ± 3.29), pain (-0.85 ± 2.48), fatigue (-0.73 ± 2.13), MDHAQ PGA (-0.72 ± 2.39), and MDHAQ PtGA (-0.53 ± 2.62) (Table 2). The improvements at 3 months were maintained at 6 months.
Conclusion: TNF inhibitor-experienced patients with PsA who initiated UPA demonstrated significant and sustained improvements in joint involvement, pain, fatigue, and overall health in real-world clinical practice.
Reference:
1. Mease PJ, et al. Ann Rheum Dis. 2021;80:312-20.
Disclosures: A. Ogdie: AbbVie, 2, 5, Amgen, 2, 5, Bristol-Myers Squibb(BMS), 5, Celgene, 2, CorEvitas, 2, Eli Lilly, 2, Gilead, 2, GlaxoSmithKlein(GSK), 5, Happify Health, 2, Janssen, 2, 5, Novartis, 2, 5, Pfizer, 2, 5, UCB, 2; X. Ye: AbbVie, 3, 11; Y. Peng: AbbVie, 3, 11; C. Saffore: AbbVie, 3, 11; J. Stigler: AbbVie, 3, 11; M. Bergman: AbbVie, 2, 6, Amgen, 2, 6, Bristol-Myers Squibb(BMS), 2, 6, GlaxoSmithKlein(GSK), 2, 6, Janssen, 2, 6, Johnson & Johnson, 11, Merck/MSD, 2, 6, 11, Novartis, 2, 6, Pfizer, 2, 6, Sa, 2, 5, Sandoz, 2, 6, Scifer, 2, 6.
To cite this abstract in AMA style:
Ogdie A, Ye X, Peng Y, Saffore C, Stigler J, Bergman M. Real World Effectiveness of Upadacitinib in Patients with Psoriatic Arthritis Previously Treated with TNF Inhibitors: Data from the OM1 Registry [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/real-world-effectiveness-of-upadacitinib-in-patients-with-psoriatic-arthritis-previously-treated-with-tnf-inhibitors-data-from-the-om1-registry/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/real-world-effectiveness-of-upadacitinib-in-patients-with-psoriatic-arthritis-previously-treated-with-tnf-inhibitors-data-from-the-om1-registry/