Session Information
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: COMPACT is a multi-country, non-interventional study that evaluated the drug persistence, effectiveness, safety and patient-reported outcomes in patients with rheumatoid arthritis (RA), axial spondyloarthritis and psoriatic arthritis treated with GP2015 (an etanercept biosimilar) in real-world conditions. Here, we report the drug persistence of GP2015 in patients with RA after 12 months.
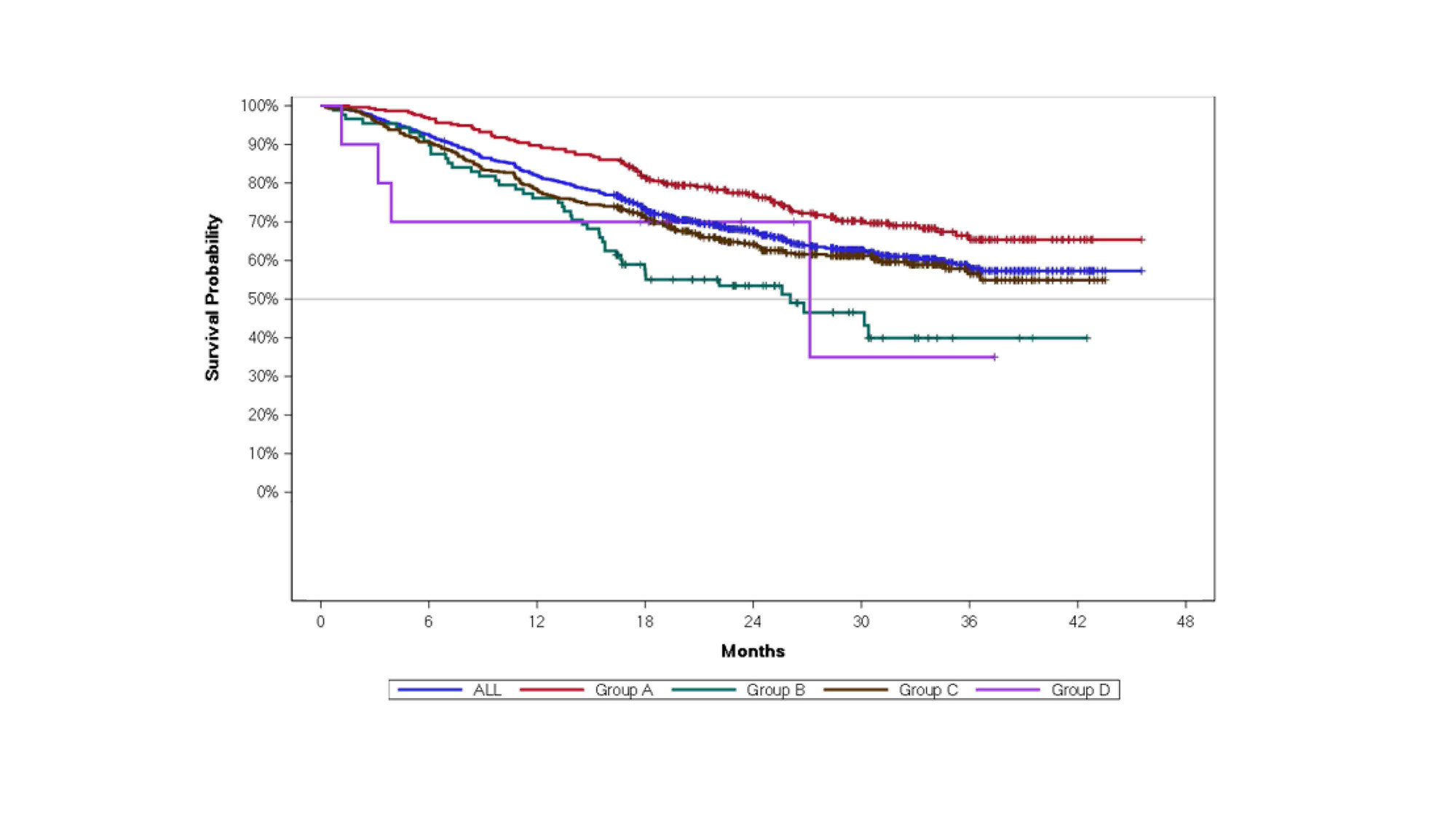
Methods: RA patients aged ≥18 years on GP2015 treatment prior to study were enrolled. Patients were categorized based on prior treatment status: patients in clinical remission or low disease activity under treatment with reference etanercept (ETN) or other biosimilar ETN (initial ETN: [iETN]) and switched to GP2015 (Group A) or patients who received non-ETN targeted therapies and switched to GP2015 (Group B) or biologic-naïve patients who started GP2015 as the first biologic treatment after conventional therapy failure (Group C) or DMARD-naïve patients with recent diagnosis of RA considered suitable for treatment initiation with a biologic and started on treatment with GP2015 (Group D). Patients’ drug persistence from GP2015 treatment start was assessed and is presented here. Median drug persistence was calculated using the median time estimate from Kaplan-Meier curve.
Results: Of 1466 patients enrolled, 844 (57.6%) patients had RA reported as the primary indication. Of the 844 patients with RA, 295 patients were switched from iETN (Group A), 88 were switched from other targeted therapies (Group B), 451 were biologic-naïve (Group C), and 10 were DMARD-naïve (Group D).1 Drug persistence from start of GP2015 treatment in RA patients is presented in Figure 1. In the overall RA population, the discontinuation rate was 18.0% at Month 12 after initiation of GP2015 treatment. At Month 12, patients in Group A reported the lowest rate of discontinuation of 10.2%, while patients in Group B and C reported discontinuation rates of 23.9%, and 21.8%, respectively. Due to limited patient numbers in Group D (n=10) the drug persistence rate was difficult to interpret.
Conclusion: The results of the study show high treatment persistence under GP2015 treatment in RA patients under real-world conditions. The results are in line with the reported persistence rates in the overall study population. Patients who were on previous iETN treatment and who had stable disease presented the highest drug survival rates after switch to GP2015. Reference: 1.Schmalzing, et al. Ann Rheum Dis. 2022;81(Suppl 1):589–90.
To cite this abstract in AMA style:
Schmalzing M, Both C, Brueckmann I, Santos J, Sheeran T, Kellner H, Askari A. Real-World Drug Persistence of GP2015, an Etanercept Biosimilar, in Patients with Rheumatoid Arthritis: Results from the Multi-Country COMPACT Study [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/real-world-drug-persistence-of-gp2015-an-etanercept-biosimilar-in-patients-with-rheumatoid-arthritis-results-from-the-multi-country-compact-study/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/real-world-drug-persistence-of-gp2015-an-etanercept-biosimilar-in-patients-with-rheumatoid-arthritis-results-from-the-multi-country-compact-study/