Session Information
Date: Friday, March 31, 2023
Title: Plenary Abstracts Session II
Session Type: Plenary Session
Session Time: 2:30PM-3:00PM
Background/Purpose: The morbidity of chronic glucocorticoid (GC) use is rarely captured as a standardized clinical outcome in pediatric rheumatic conditions. The newly developed pediatric glucocorticoid toxicity index (pGTI) (Brogan et al. 2022) presents a novel opportunity to operationalize measurement of GC toxicity for research and clinical care. We tested the feasibility and construct validity of the pGTI in our pediatric lupus nephritis (pLN) cohort.
Methods: We conducted a retrospective cohort study of patients with pLN in the Boston Childrens Hospital Lupus Registry with rheumatology visits since January 2016 and ³6 months of systemic GC treatment. The index visit was the visit date at which GC were initiated. We scored steroid toxicity items in 12/15 domains at visits occurring every 6 months (+/-2) for up to 3 years, with positive scores indicating new/worsening toxicity and negative scores indicating improvement. Scores were collected prospectively for skin and neuropsychiatric toxicity domains as of November 2022; the remainder were abstracted via manual chart review. We calculated total pGTI for each visit interval, Cumulative Worsening Score (CWS) and Aggregate Improvement Score (AIS). Pearsons correlation coefficients and mixed effects linear regression for repeated measures were used to evaluate relationships between daily, average, or cumulative prednisone dose and respective pGTI indices.
Results: We included 227 visits by 45 patients (median of 5 visits/patient [IQR 2-4]) (Table 1). Glucose metabolism (hemoglobin A1c), hyperlipidemia, and bone mineral density domains were dropped due to infrequent clinical assessment ( 7 observations).
The largest increases in toxicity occurred in the first 6 months, but 33% of patients accrued new/worsening toxicity beyond 12 months (Fig 1). Median CWS was 14 (IQR [0-33]; N = 45) at month 6, 18 ([0-52], N = 40) at month 12, and 30 (IQR [0-53], N = 38) at month 24. The most common toxicity (51% of patients) was worsening blood pressure, including hypertensive emergency (11%) and posterior reversible encephalopathy syndrome (9%). Other common toxicities were striae, increasing body mass index (BMI), and mood disturbance (Fig 2).
pGTI score correlated modestly with current (r2 0.25, p 0.01) and interval-averaged (r2 0.27, p 0.01) prednisone dose (mg/day). There was modest correlation between cumulative dose (mg) and CWS (r2 0.41, p 0.01). In repeated measures analysis adjusted for time, cumulative dose associated with greater CWS (7 unit increase in CWS for every 100 mg higher cumulative prednisone dose, 95% CI [3.8 – 10.9]), but there was large between-subject variance.
Conclusion: Patients with pLN exhibit significant GC toxicity, particularly in the blood pressure, skin, mood and BMI domains. Calculation of the pGTI using real-world data was generally feasible, but required adaptations for missing clinical data. Modest correlation between GC dose and pGTI supports construct validity of this tool but also highlights between-subject variability in toxicity. As a standardized, pediatric-specific tool for assessing GC toxicity, the pGTI can be considered
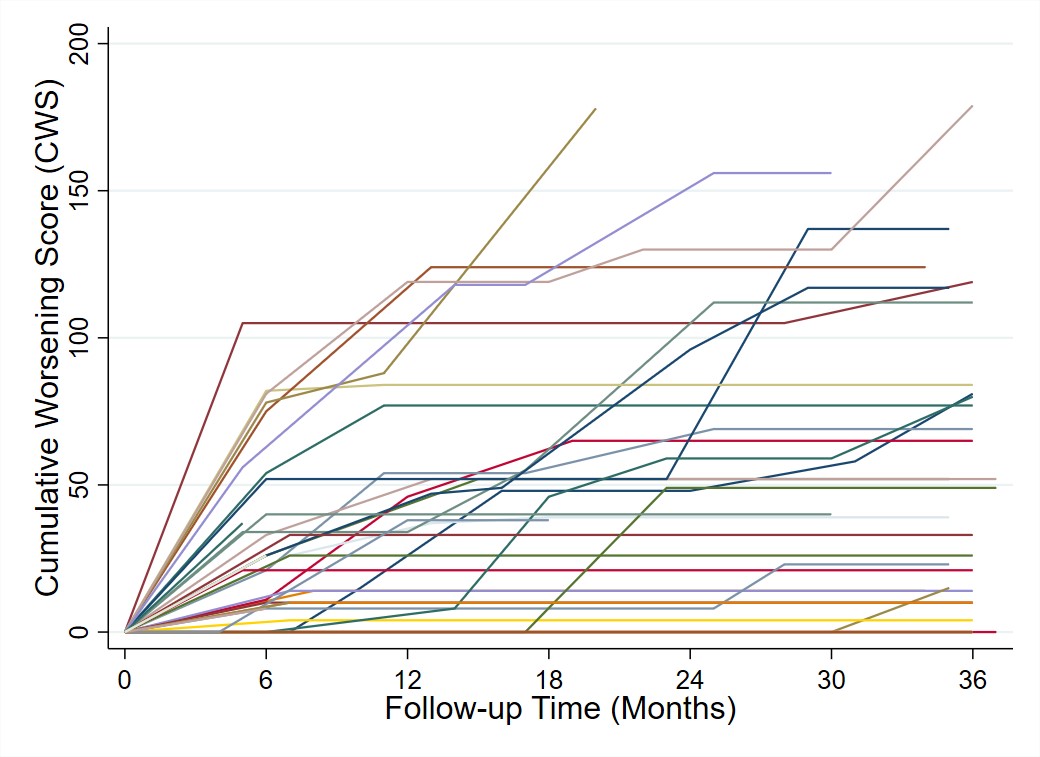 Figure 1. Cumulative Worsening Score (CWS) trajectories over three years of follow-up. CWS is a continuous summation of any toxicity that has been accrued after the index visit. Each line represents the score trajectory of an individual patient. Following discontinuation of GCs, if there was no glucocorticoid use during subsequent visit intervals, then further changes in toxicity domains could not be reliably attributed to glucocorticoid use and therefore did not contribute to the CWS.
Figure 1. Cumulative Worsening Score (CWS) trajectories over three years of follow-up. CWS is a continuous summation of any toxicity that has been accrued after the index visit. Each line represents the score trajectory of an individual patient. Following discontinuation of GCs, if there was no glucocorticoid use during subsequent visit intervals, then further changes in toxicity domains could not be reliably attributed to glucocorticoid use and therefore did not contribute to the CWS.
 Figure 2. Proportion of pediatric lupus nephritis (pLN) patients (Nf45) with new or worsening toxicity for each pediatric Glucocorticoid Toxicity item at any time during follow-up. Neuropsychiatric and skin toxicity items were captured prospectively for 4/227 (2%) of visits and otherwise abstracted from medical records.
Figure 2. Proportion of pediatric lupus nephritis (pLN) patients (Nf45) with new or worsening toxicity for each pediatric Glucocorticoid Toxicity item at any time during follow-up. Neuropsychiatric and skin toxicity items were captured prospectively for 4/227 (2%) of visits and otherwise abstracted from medical records.
To cite this abstract in AMA style:
Zhang E, Alonzi G, Hlobik M, Meidan E, Lo M, Halyabar O, Hazen M, Cohen E, Henderson L, Case S, Chang M, Frank C, Daga A, Hausmann J, Bakhsh A, Kim L, Ibanez D, Wobma H, Chandler M, Dedeoglu F, Sundel R, Nigrovic P, Costenbader K, Son M, Chang J. Real-World Application of the Pediatric Glucocorticoid Toxicity Index in Children with Lupus Nephritis: A Feasibility and Initial Validation Study [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 4). https://acrabstracts.org/abstract/real-world-application-of-the-pediatric-glucocorticoid-toxicity-index-in-children-with-lupus-nephritis-a-feasibility-and-initial-validation-study/. Accessed .« Back to 2023 Pediatric Rheumatology Symposium
ACR Meeting Abstracts - https://acrabstracts.org/abstract/real-world-application-of-the-pediatric-glucocorticoid-toxicity-index-in-children-with-lupus-nephritis-a-feasibility-and-initial-validation-study/

