Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Complement C1s is one of the major components of the classical complement pathway (CP), and the abnormal activation of CP is implicated in several autoimmune diseases. RAY121 is a novel anti-human C1s monoclonal antibody, which shows a longer half-life based on Sequential Monoclonal Antibody Recycling Technology – Immunoglobulin (SMART-Ig®) that enables a single antibody molecule to bind to an antigen multiple times. A Phase 1a, First in Human (FIH) placebo-controlled, double-blind, single ascending dose trial was designed and conducted in healthy adults.
Methods: The objectives of the FIH study were to evaluate safety, pharmacokinetics (PK), pharmacodynamics (PD), and immunogenicity after a single intravenous (IV) and subcutaneous (SC) dose of RAY121 in healthy adults. The study was consisted of five cohorts in total with IV (low, middle, and high dose) and two SC (middle and high dose) cohorts. A total of 40 healthy adults received study drug administration (RAY121 or placebo).
Results: RAY121 was well-tolerated and there was no death or serious adverse events (AEs). AEs were reported in 60.0% (18/30 subjects) of those who received RAY121, whereas 30.0% (3/10 subjects) of those who received placebo, with no dose dependency.
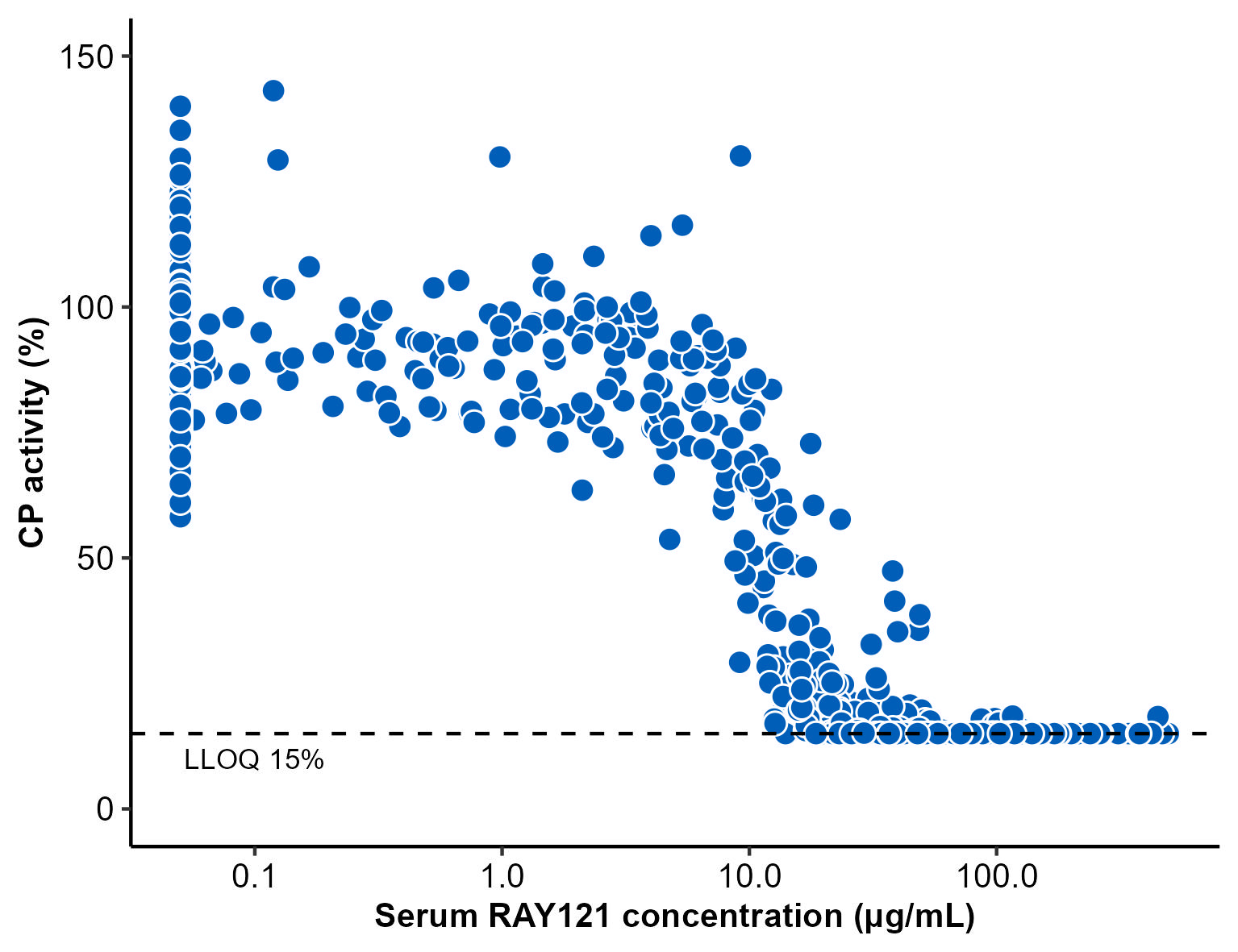
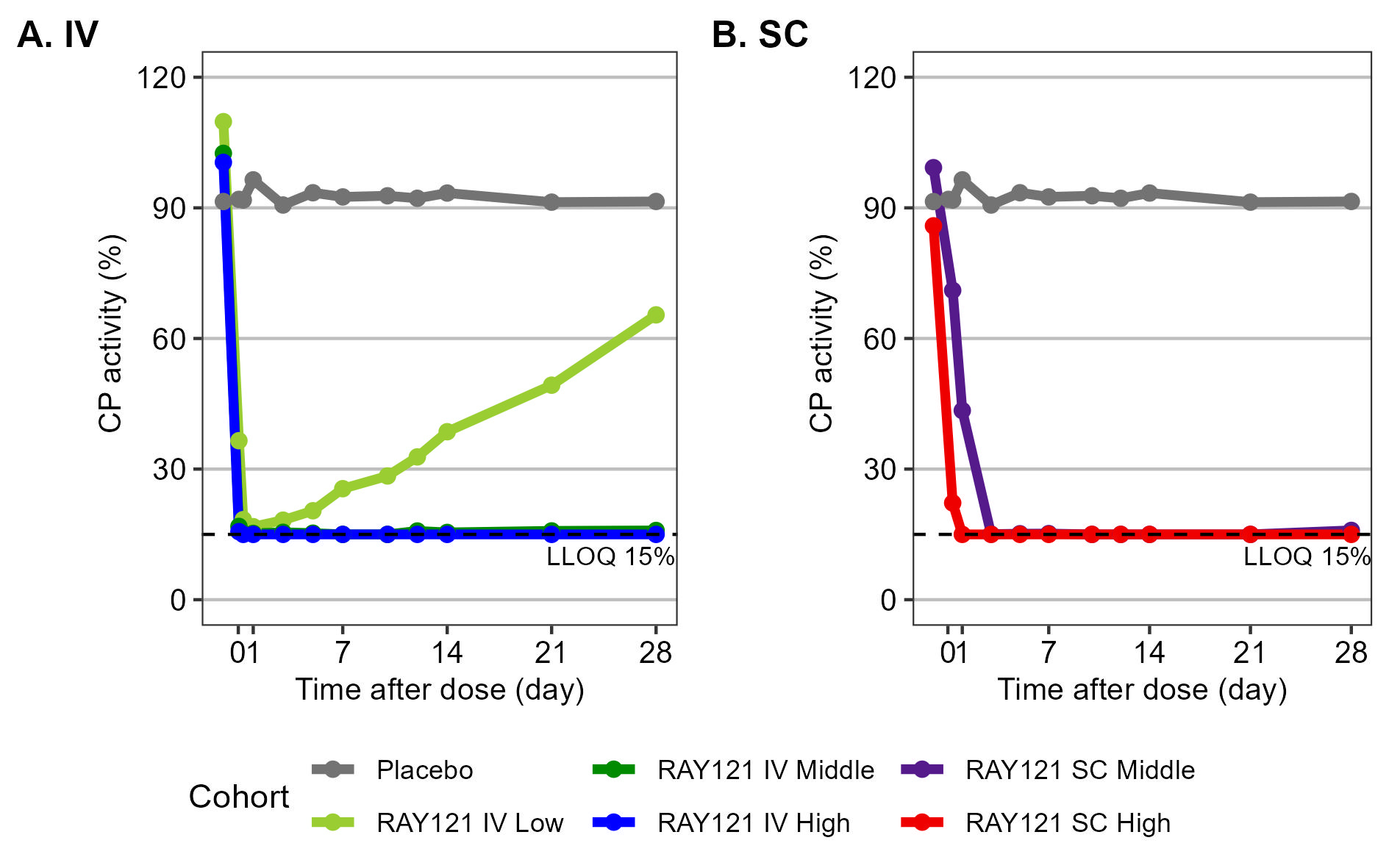
For PK profiles, the systemic exposure to RAY121 dose-dependently increased and sustained for longer time than conventional monoclonal antibodies with a half-life of 41.2 days. Furthermore, RAY121 suppressed CP activity in a concentration-dependent manner (Figure 1) and CP activity remained completely suppressed at 4 weeks after a single dose of RAY121 at 4.5 mg/kg or more (Figure 2).
Conclusion: In summary, the FIH study of RAY121 demonstrated that the application of Recycling Antibody engineering has been indeed translated into the substantial extension of the half-life of serum drug concentrations and the prolonged efficient suppression of CP with the favorable safety and PK profile. As a result, it has been hypothesized that infrequent SC dose of RAY121 enables maintaining complete suppression of classical complement pathway. The characteristic of monthly SC injection of RAY121 has a significant clinical value and is expected to be a new therapy, in reducing clinical burden on patients with autoimmune diseases.
CP, classical complement pathway; LLOQ, lower limit of quantification.
IV, intravenous; SC, subcutaneous; CP, classical complement pathway; LLOQ, lower limit of quantification.
To cite this abstract in AMA style:
Haranaka M, Yamada A, Takahashi S, Gotanda K, Nakano-Kanatani S, Uemura N, Ohnishi K, Nishidate M, Ohnami I, Kai M. RAY121, a Novel Recycling Monoclonal Antibody Against Complement C1s: Safety, Pharmacokinetic and Pharmacodynamic Data from a Phase 1a First in Human Clinical Trial in Healthy Adults [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/ray121-a-novel-recycling-monoclonal-antibody-against-complement-c1s-safety-pharmacokinetic-and-pharmacodynamic-data-from-a-phase-1a-first-in-human-clinical-trial-in-healthy-adults/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/ray121-a-novel-recycling-monoclonal-antibody-against-complement-c1s-safety-pharmacokinetic-and-pharmacodynamic-data-from-a-phase-1a-first-in-human-clinical-trial-in-healthy-adults/