Session Information
Date: Sunday, November 12, 2023
Title: (0609–0672) Systemic Sclerosis & Related Disorders – Clinical Poster I: Research
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Individuals with self-identified Black race have a higher incidence of systemic sclerosis (SSc), develop SSc at a younger age, and have a more severe clinical phenotype than their White counterparts. To understand the roles autoantibodies may play in driving racial variation in disease severity, we compared autoantibody distribution and associated phenotypes between cohorts of Black and White individuals from the US multicenter Genome Research in African American Scleroderma Patients (GRASP) cohort and the Johns Hopkins Scleroderma Center cohort, respectively.
Methods: 803 Black and 2178 White patients with SSc had systematic autoantibody testing for anti-centromere (ACA), anti-RNA-polymerase III (POLR3), anti-Scl70, anti-PMSCL, anti-NOR90, anti-ThTo, anti-Ku, anti-U3RNP, and anti-Ro52 using the Euroimmun platform and anti-U1RNP using a commercial ELISA assay. 93.7% and 94.2% of individuals in the Black and White cohorts met 2013 ACR/EULAR criteria for SSc. Autoantibody frequency was compared between the two groups. To assess the effect of autoantibodies on the association between race and clinical outcomes, odds ratio coefficients for race from multivariable models including and excluding autoantibodies were compared. Multivariable logistic regression analyses were performed to assess the association between each autoantibody and clinical outcomes. Clinical outcomes included common manifestations of SSc, and organ-specific disease severity.
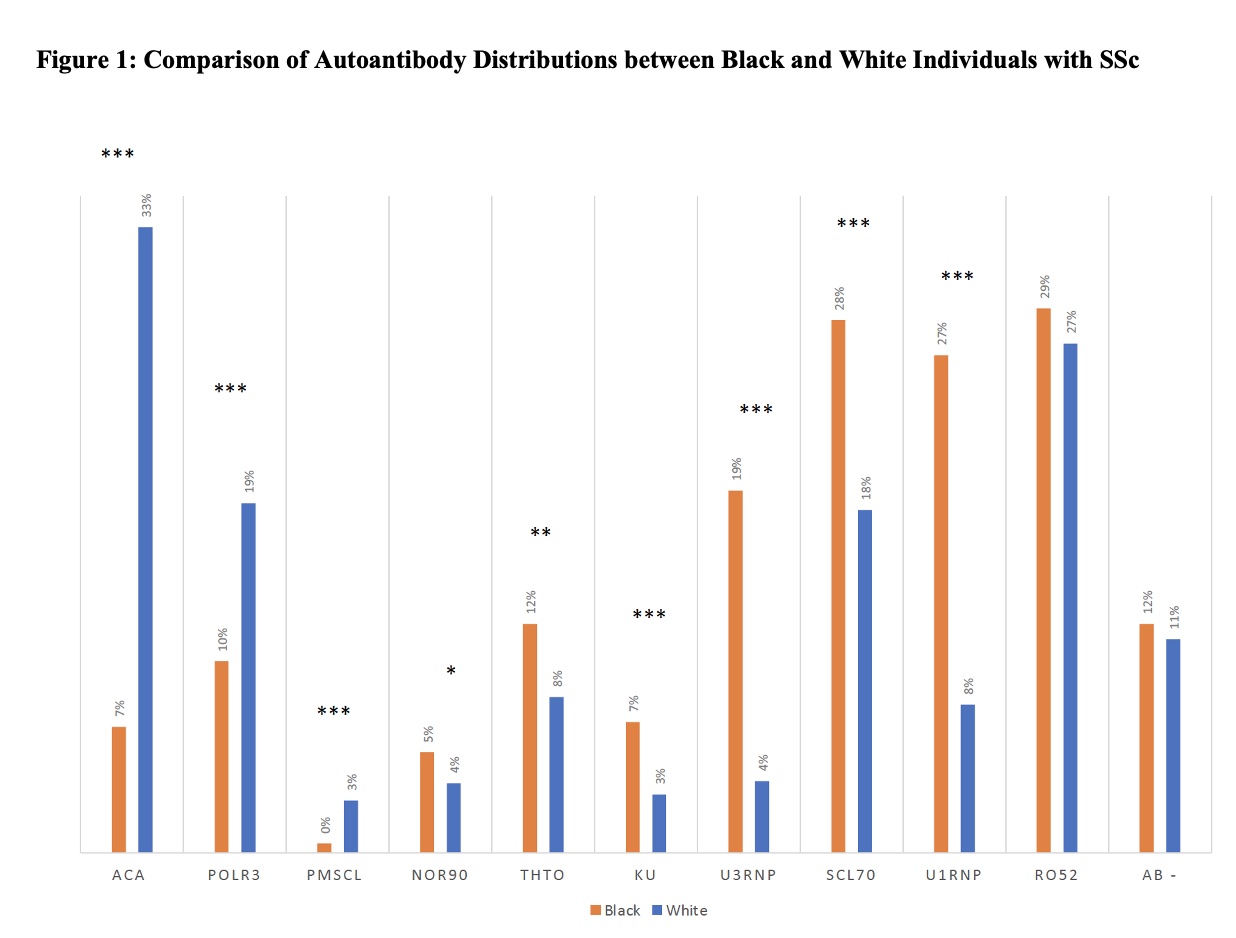
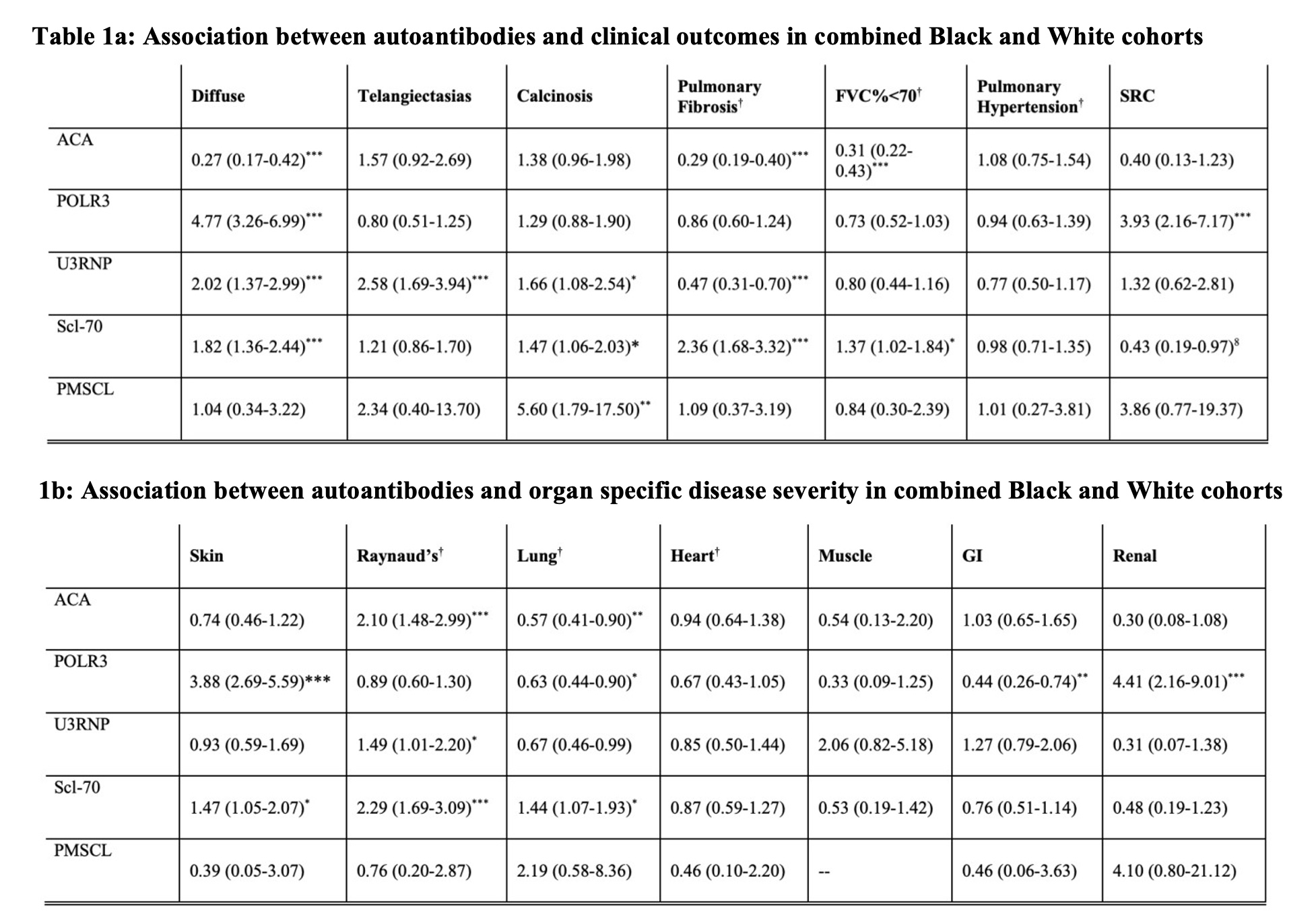
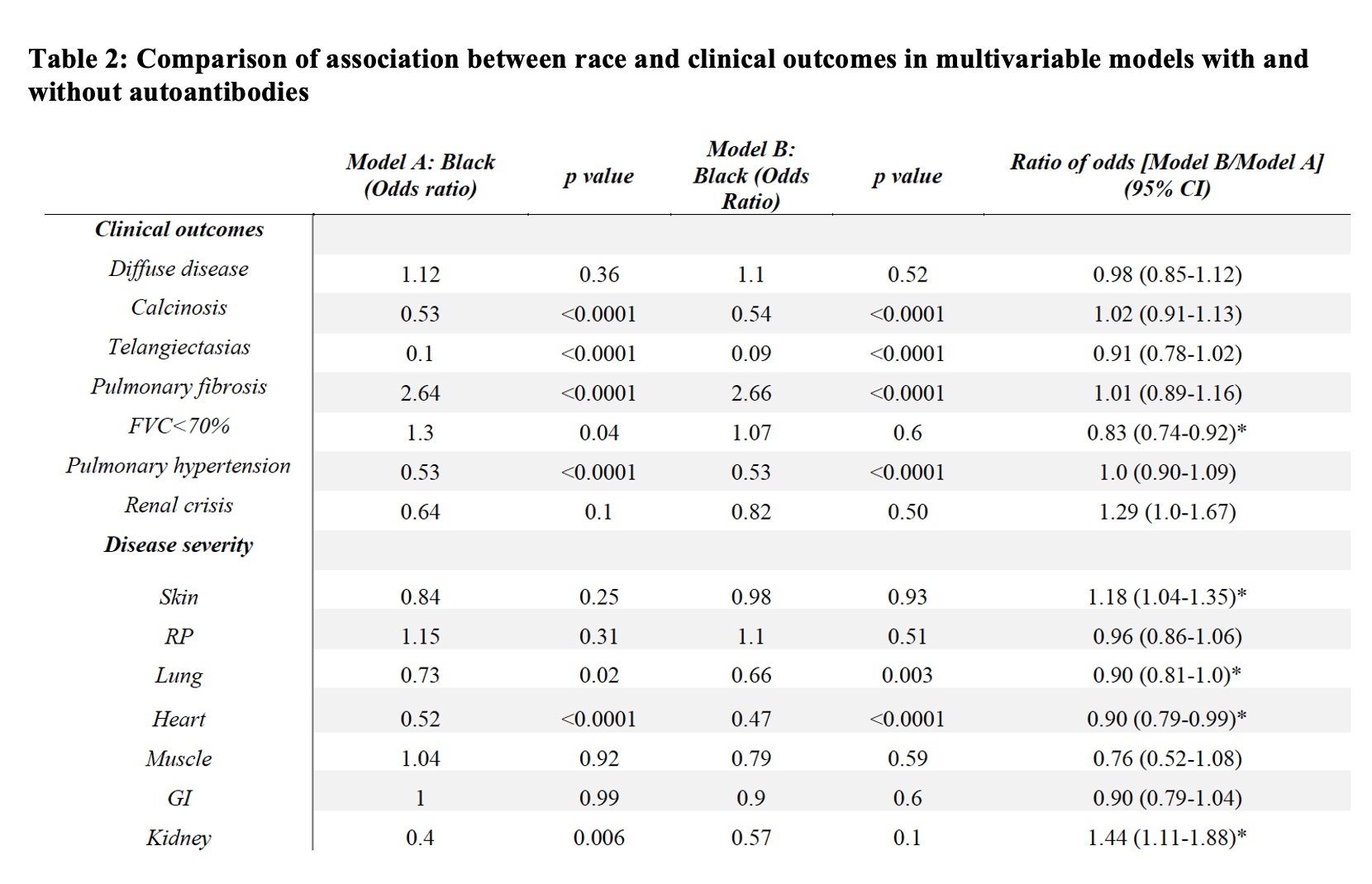
Results: Comparing these self-reported racial groups, the Black cohort had a higher mean modified Rodnan skin score and higher rates of pulmonary fibrosis; Black individuals had higher rates of severe Raynaud’s phenomenon, skin, gastrointestinal and lung disease. Statistically significant differences were seen in autoantibody distribution between the Black and White cohorts (Figure 1). Among the Black cohort, anti-Scl70 (28%), anti-U1RNP (27%), and anti-U3RNP (19%) were most common while the White cohort was enriched for ACA (33%) and anti-POLR3 (19%). In multivariable models, anti-Scl70 was associated with diffuse skin disease, severe cutaneous disease, pulmonary fibrosis and severe lung disease (Table 1). Anti-U3RNP was associated with diffuse skin disease, telangiectasias, calcinosis, and severe Raynaud’s phenomenon. Anti-POLR3 was associated with severe skin and renal disease. Adjusting for autoantibodies decreased the effect of race on clinical outcome by 17%, 10% and 10% for FVC< 70%, severe lung and heart disease respectively; it increased the effect of race by 18% for severe skin and 44% for severe kidney disease (Table 2).
Conclusion: This study is the largest systematic analysis of autoantibody responses and associated clinical phenotypes in a geographically diverse population of Black individuals with SSc. Black and White individuals with SSc had distinct autoantibody distributions. Antibodies common in the Black cohort (anti-Scl70, anti-U1RNP, anti-U3RNP, anti-ThTo) commonly associate with severe skin disease and interstitial lung disease. However, differences in autoantibody distributions explain only a small fraction of the racial effects on clinical outcomes for FVC< 70%, severe lung and heart disease.
* p<0.05, ** p<0.005, *** p<0.0005.
* p<0.05, ** p<0.005, *** p<0.0005
† Covariate of smoking status (ever versus never smoked cigarettes was included in the multivariate analysis)
* statistical significance
To cite this abstract in AMA style:
Kuchinad K, Kim J, Woods A, Leatherman G, Gutierrez L, Mayes M, Domsic R, Ramos P, Silver R, Varga J, Saketkoo L, Kafaja S, Shanmugam V, Gordon J, Chung L, Bernstein E, Gourh P, Boin F, Kastner D, Zeger S, Casciola-Rosen L, Wigley F, Shah A. Racial Variability in Immune Responses Only Partially Explains Differential Systemic Sclerosis Disease Severity [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/racial-variability-in-immune-responses-only-partially-explains-differential-systemic-sclerosis-disease-severity/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/racial-variability-in-immune-responses-only-partially-explains-differential-systemic-sclerosis-disease-severity/