Session Information
Date: Sunday, November 8, 2015
Title: Rheumatoid Arthritis - Human Etiology and Pathogenesis Poster I
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose:
Antibodies against citrullinated proteins (ACPAs) are the
autoimmune hallmark of rheumatoid arthritis (RA), and can precede the onset of
symptomatic disease by years. By introducing neo-epitopes into self-proteins, posttranslational
protein citrullination is thought to break immune tolerance and drive
autoimmunity in RA. While many RA autoantigens are recognized in a
citrulline-dependent context, some antigens are targeted as native (unmodified)
proteins in RA, including Fc-gamma (targeted by rheumatoid factor), hnRNP A2/B1
(RA33), peptidylarginine deiminase
type 4 (PAD4) and calpastatin among others. How these
antibody systems fit into the current paradigm of autoantigen citrullination in
RA is not known.
Methods:
Synovial fluid (SF) cell samples from patients with RA were
analyzed by mass spectrometry (MS) and immunoblotting. HnRNP A2/B1/A2b/B1b
(RA33) was cloned from RA SF cell cDNA, and expressed as recombinant proteins
in an E coli system. Purified RA33 splicing variants were citrullinated
in vitro using human rPAD4. Patients with RA (n=196 from the baseline visit of
a prospective cohort study) and healthy controls (n=56) were assayed in
parallel for antibodies against native and citrullinated hnRNP B1b by
quantitative ELISA. Patients fulfilled the American College of Rheumatology
1987 revised criteria for the classification of RA. Antibody specificity
against native and citrullinated RA33 was further analyzed by immunoblotting
and immunoprecipitation (IP) using in vitro
transcribed-translated, radiolabeled RA33.
Results:
We found that RA33, a classic native autoantigen in RA, is
citrullinated in the rheumatoid joint. MS analysis of RA SF cells and in-vitro
citrullinated RA33 confirmed citrullination in 16/25 arginine residues of the
full-length protein. Using native and citrullinated antigen, we demonstrated
that RA33 is targeted by patient sera in three ways: only as a native protein;
both as a native and as a citrullinated protein (double-reactive sera); and
only as a citrullinated protein. These distinct patterns of reactivity were
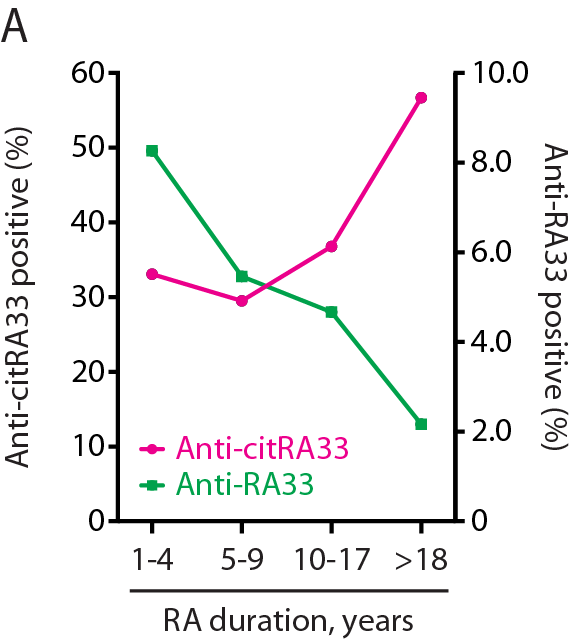
confirmed by IP, immunoblotting and competition assays. Anti-(native) RA33
antibodies were almost exclusively detected in early RA (Figure A). In
contrast, anti-citRA33 antibodies were positively associated with disease
duration, and most commonly detected in patients with long established disease
(>18 years post diagnosis). The unique subset of double-reactive patients
appeared to identify a transitional disease phase in the evolution of an
exclusively anti-native to a mature anti-citrullinated protein antibody
response in RA.
Conclusion:
The study of RA33 suggests that native and citrullinated
proteins targeted by autoantibodies in RA are part of a single antibody system,
which appears to evolve with disease duration. This challenges the paradigm of
citrullination as the inciting principle underlying loss of tolerance in RA.
To cite this abstract in AMA style:
Konig MF, Giles J, Nigrovic PA, Andrade F. RA33 Challenges the Paradigm of Autoantigen Selection in Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2015; 67 (suppl 10). https://acrabstracts.org/abstract/ra33-challenges-the-paradigm-of-autoantigen-selection-in-rheumatoid-arthritis/. Accessed .« Back to 2015 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/ra33-challenges-the-paradigm-of-autoantigen-selection-in-rheumatoid-arthritis/