Session Information
Date: Sunday, October 26, 2025
Title: (0671–0710) Systemic Sclerosis & Related Disorders – Clinical Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Calcinosis cutis (CC) is a disabling skin condition associated with systemic sclerosis (SSc). Although many CC treatments including sodium thiosulfate (STS) have been proposed, no clinical trials have been conducted due, in part, to a lack of validated quantitative outcome measures.
Methods: Patients with severe SSc-CC, enrolled in an open-label case series were treated with 25% STS was administered topically twice daily for 6-12 months or injected intradermally monthly for six months (0.1ml per injection for total of 1-2.5ml STS injected per session) to a patient-selected region-of-interest (ROI). All patients satisfied the ACR classification criteria of SSc. Three clinical coordinators independently assessed computer-assisted annotation of SSc-CC on computed tomography (CT) exams for quantitative measurement of volume changes compared to symptomatic and photographic responses during STS treatment using BioImage Suite Web software. Inter-rater agreement and test-retest reliability were determined with intraclass coefficient (ICC).
Results: Five women, with SSc-CC involving the upper arm, bilateral ischial tuberosities, hands (two patients) and bilateral patellae, received topical (n=2) or intralesional (n=3) STS. Variable changes in symptoms, digital camera imaging, and CC volume by CT imaging (28% reduction in upper arm; 75% and 10% reductions in left and right, buttock, respectively; 18% increase in left forefinger; 27% increase in right hand; and 12% reduction in left, and 4% increase in right, patellae) were observed. Inter-rater agreement was excellent with an ICC of 0.93. Volume of CC on repeated measures performed one week apart was within 3%. Annotation time ranged from < 30 minutes for arm and buttock to >four hours per case for other ROI.
Conclusion: Intradermal STS treatment was associated with symptomatic and volumetric improvement in arm and buttocks, but not finger CC. Topical STS was not associated with symptomatic or imaging CC improvement in fingers or patellae. Computer-assisted annotation and volumetric measurement of CC on CT imaging appears to be a reproducible and sensitive quantitative outcome. However, time to annotate CC close to bone was lengthy due to similar radiographic density. Computer-assisted quantification of CC on CT is a useful quantitative outcome for future clinical trials, but additional methods for automating CC annotation need to be developed.
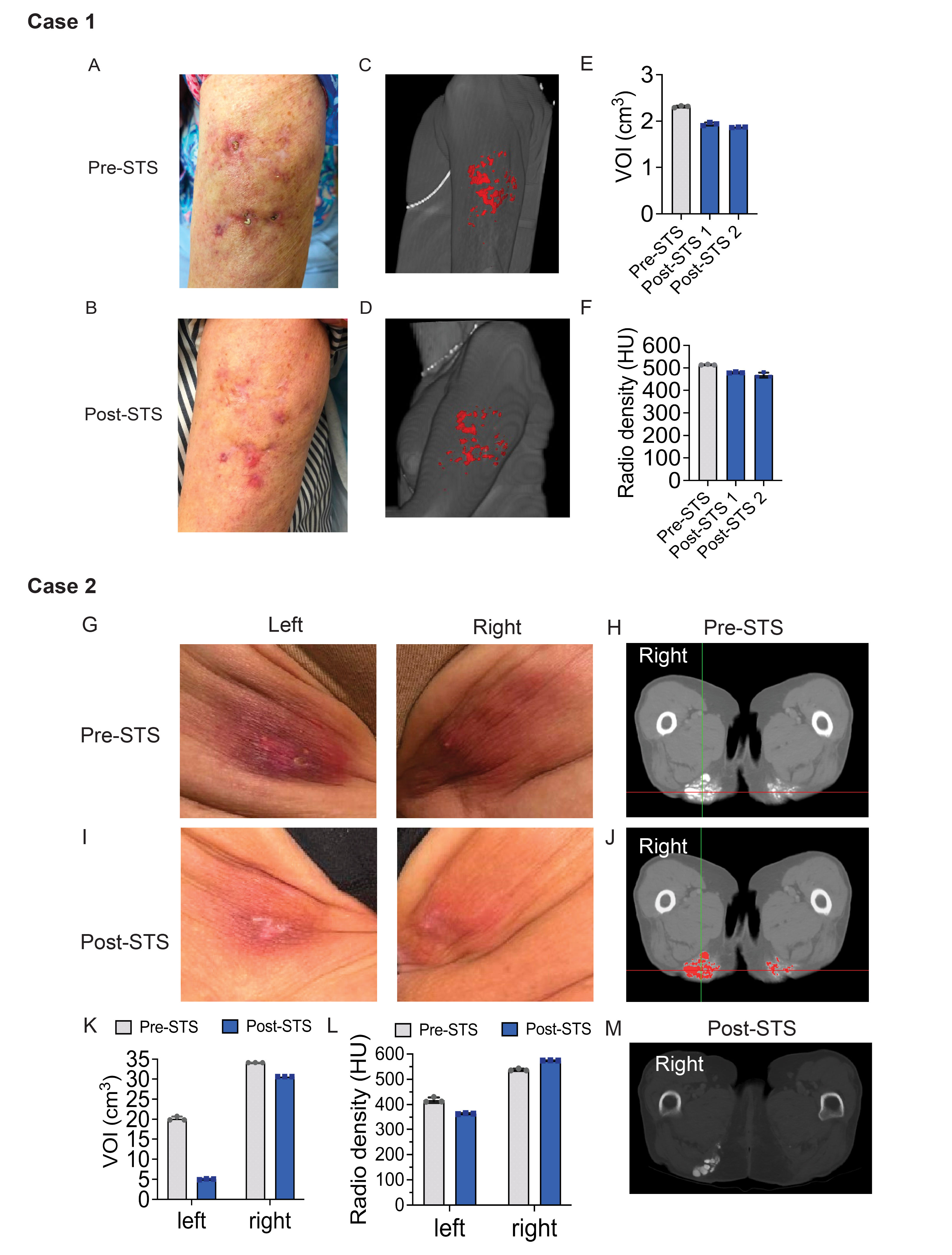 Figure 1. Intralesional sodium thiosulfate treatment of arm calcinosis cutis (CC) (Case 1) and buttock CC (Case 2). (A, B) Pre- and post-STS treatment photographs of left upper arm, respectively. (C, D) Rasterized pre- and post-STS treatment computed tomography (CT) images of CC lesions, respectively, with CC painted red. (E). Pre- and post-STS treatment CC lesional volume quantification. Post-STS treatment CT exams 1 and 2 were repeated one week apart, VOI=volume of interest. (F) Average pre- and post-treatment CC lesional radio density quantification among 3 raters. (G, I) Left and right buttock photographs of pre- and post-STS treatment, respectively. (H, J) Pre- STS treatment axial CT images without and with painting of CC lesions. (K) Pre- and post-treatment CC lesional volume quantification, VOI=volume of interest. (L) Average pre- and post-treatment CC lesional radio density quantification among 3 raters. (M) Post-treatment axial CT image without painting of CC lesions.
Figure 1. Intralesional sodium thiosulfate treatment of arm calcinosis cutis (CC) (Case 1) and buttock CC (Case 2). (A, B) Pre- and post-STS treatment photographs of left upper arm, respectively. (C, D) Rasterized pre- and post-STS treatment computed tomography (CT) images of CC lesions, respectively, with CC painted red. (E). Pre- and post-STS treatment CC lesional volume quantification. Post-STS treatment CT exams 1 and 2 were repeated one week apart, VOI=volume of interest. (F) Average pre- and post-treatment CC lesional radio density quantification among 3 raters. (G, I) Left and right buttock photographs of pre- and post-STS treatment, respectively. (H, J) Pre- STS treatment axial CT images without and with painting of CC lesions. (K) Pre- and post-treatment CC lesional volume quantification, VOI=volume of interest. (L) Average pre- and post-treatment CC lesional radio density quantification among 3 raters. (M) Post-treatment axial CT image without painting of CC lesions.
.jpg) Figure 2. Intralesional (Case 3) and topical (Case 4) sodium thiosulfate treatment of finger calcinosis cutis (CC). (A, B) Pre- and post-STS treatment photographs of the right index finger, respectively. (C) Pre-STS treatment axial CT images without and with painting of CC lesions. (D) Post-treatment axial CT images without and with painting of CC lesions. (E) Average pre- and post-treatment CC lesional volume quantification among 3 raters, VOI=volume of interest. (F) Average pre- and post-treatment CC lesional radio density quantification among 3 raters. (G) Average pre- and post-treatment CC lesional SCTC scores among 3 raters. (H) Pre-STS treatment axial CT images without and with painting of CC lesions. (I) Post-treatment axial CT images without and with painting of CC lesions. (J) Average pre- and post-treatment CC lesional volume quantification among 3 raters, VOI=volume of interest. (K) Average pre- and post-treatment CC lesional radio density quantification among 3 raters. (L) Average pre- and post-treatment CC lesional SCTC scores among 3 raters.
Figure 2. Intralesional (Case 3) and topical (Case 4) sodium thiosulfate treatment of finger calcinosis cutis (CC). (A, B) Pre- and post-STS treatment photographs of the right index finger, respectively. (C) Pre-STS treatment axial CT images without and with painting of CC lesions. (D) Post-treatment axial CT images without and with painting of CC lesions. (E) Average pre- and post-treatment CC lesional volume quantification among 3 raters, VOI=volume of interest. (F) Average pre- and post-treatment CC lesional radio density quantification among 3 raters. (G) Average pre- and post-treatment CC lesional SCTC scores among 3 raters. (H) Pre-STS treatment axial CT images without and with painting of CC lesions. (I) Post-treatment axial CT images without and with painting of CC lesions. (J) Average pre- and post-treatment CC lesional volume quantification among 3 raters, VOI=volume of interest. (K) Average pre- and post-treatment CC lesional radio density quantification among 3 raters. (L) Average pre- and post-treatment CC lesional SCTC scores among 3 raters.
.jpg) Figure 3. Topical sodium thiosulfate treatment of knee calcinosis cutis (CC). (A, B) Left and right patellae photographs of pre- and post-STS treatment, respectively. (C) Pre- STS treatment axial CT images without painting of CC lesions. (D) Post- STS treatment axial CT images of right patella without painting of CC lesions. (E) Average pre- and post-treatment CC lesional volume quantification among 3 raters, VOI=volume of interest. (F) Average pre- and post-treatment CC lesional radio density quantification among 3 raters. (G) Pre-STS treatment axial CT image with painting of CC lesion.
Figure 3. Topical sodium thiosulfate treatment of knee calcinosis cutis (CC). (A, B) Left and right patellae photographs of pre- and post-STS treatment, respectively. (C) Pre- STS treatment axial CT images without painting of CC lesions. (D) Post- STS treatment axial CT images of right patella without painting of CC lesions. (E) Average pre- and post-treatment CC lesional volume quantification among 3 raters, VOI=volume of interest. (F) Average pre- and post-treatment CC lesional radio density quantification among 3 raters. (G) Pre-STS treatment axial CT image with painting of CC lesion.
To cite this abstract in AMA style:
Odell I, Cheung C, Wu M, Perez S, Dixit A, van Horn C, Hamdan M, Gunes B, Kujawski S, Lee H, Wang A, Esserman D, Zamani M, Wilson F, Onofrey J, Papademetris X, Hinchcliff M. Quantitative Imaging in Systemic Sclerosis Patients Receiving Sodium Thiosulfate for Calcinosis Cutis [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/quantitative-imaging-in-systemic-sclerosis-patients-receiving-sodium-thiosulfate-for-calcinosis-cutis/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/quantitative-imaging-in-systemic-sclerosis-patients-receiving-sodium-thiosulfate-for-calcinosis-cutis/
