Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Sjögren’s disease (SjD) is a chronic, systemic autoimmune disease characterized by lymphocytic infiltration of the endocrine glands. Globally, SjD affects between 0.01% and 0.05% of the population, yet it may affect as many as 4 million people in the US. Ocular and oral dryness, muscle/joint pain, and fatigue are commonly reported symptoms in SjD. In this qualitative study, participants with SjD were interviewed to understand the nature and relative importance of SjD symptoms, to evaluate the content validity of the Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-Fatigue) in the context of SjD, and to assess the understandability of the symptom-specific Numeric Rating Scales (NRSs).
Methods: In June 2023, in-depth individual interviews were conducted with 15 adults in the US with self-reported clinician-diagnosed SjD not associated with other autoimmune rheumatic diseases. Participants were required to have self-reported moderate to severe oral dryness, ocular dryness, and/or muscle/joint pain in the 30 days prior to interview. Each 60-minute virtual interview was conducted in English in a hybrid (concept elicitation/cognitive debriefing) semistructured format, using an interview guide to encourage response spontaneity and to ensure consistent, systematic data collection. SjD symptoms experienced by participants were reported via concept elicitation. Participant feedback was generated using the 13-item FACIT-Fatigue scale for symptoms of fatigue and the symptom-specific NRS items for daily symptoms of ocular and oral dryness and muscle/joint pain. Regarding the ACR classification criteria for SjD, all diagnoses were self-reported as being physician diagnosed.
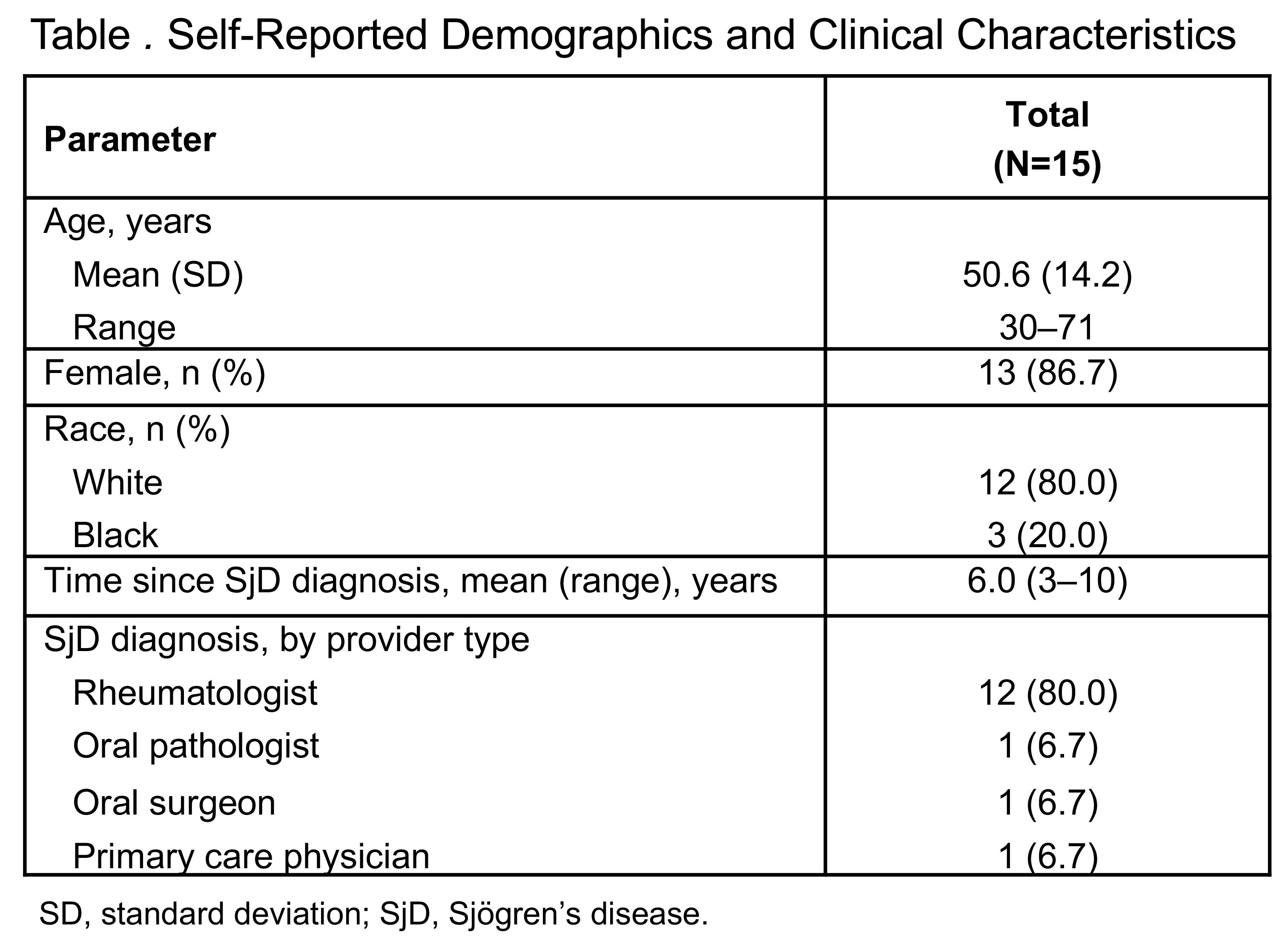
Results: The 15 participants interviewed had a mean age of 50.6 years (range: 30–71); 86.7% (n=13) were female and 80.0% (n=12) were White (Table). The mean time since clinical diagnosis of SjD was 6 years (range: 3–10). More than 85% of participants reported experiencing ocular and oral dryness, fatigue, and muscle/joint pain due to SjD; these symptoms were also reported as the most bothersome (53% ocular dryness; 40% oral dryness; 27% muscle/joint pain; 13% fatigue). Most participants (86.7%; n=13) reported that the FACIT-Fatigue scale captured all important aspects of SjD-associated fatigue. The remaining participants (n=2) identified 2 items as potentially not relevant to their own experience of fatigue. Additionally, 93.3% of participants (n=14) reported easy recall of fatigue symptoms during the previous 7 days. Debriefing results also showed that participants were able to easily understand and provide a response to symptom-specific NRS items, with no difficulties reported regarding the 24-hour recall period.
Conclusion: Interview results support the content validity of the FACIT-Fatigue scale and the relevance of symptom-specific NRS items in patients with SjD. The results also show that the FACIT-Fatigue scale and the NRS items were well understood, easily completed, and captured relevant symptoms experienced by a majority of patients with SjD, which supports the use of these patient-reported outcomes in phase 3 trials.
To cite this abstract in AMA style:
Arends S, Choi J, Becker B, Edwards T, Sreih A, Christodoulou A, Strand V. Qualitative Study on Patient-Reported Outcome Measures in Patients with Sjögren’s Disease [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/qualitative-study-on-patient-reported-outcome-measures-in-patients-with-sjogrens-disease/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/qualitative-study-on-patient-reported-outcome-measures-in-patients-with-sjogrens-disease/