Session Information
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose:
Genetic factors contribute strongly to Rheumatoid arthritis (RA) risk. Protein tyrosine phosphatase non-receptor 22 [PTPN22] encodes the hematopoietic-specific Lymphoid Phosphatase [“Lyp”]. A PTPN22 coding variant “LypW” is the most potent non-HLA genetic risk factor for RA. TLR agonists potently suppress inflammatory arthritis in murine models of RA. Our previous work revealed that Lyp promotes induction of type 1 interferon [IFN1] and IFN1-dependent genes during TLR3 agonist treatment of serum transfer arthritis (STA). RA-associated variant LypW shows loss of function behavior in arthritis suppression. The molecular and cellular mechanism(s) by which Lyp promotes IFN1-dependent inflammatory arthritis amelioration are not known. The goal of this study was to analyze the PTPn22-dependent transcriptome within arthritic synovium of animals receiving TLR agonist treatment.
Methods:
Wild-type (WT) or Lyp-deficient (Lyp-/-) mice (n = 6 per genotype) were injected with K/BxN serum on days 0 and 2. On days 4 and 6, half of the mice were given 150 μg poly I:C [pIC], a TLR 3 agonist and arthritis-suppressing agent, by intraperitoneal injection. 12 hours after the second dose of pIC, mice were sacrificed. Total RNA was extracted from ankle synovial aspirates. RNA-Seq mapping, gene quantification and differential expression were done in Bowtie2, Feature Counts and edgeR.
Results:
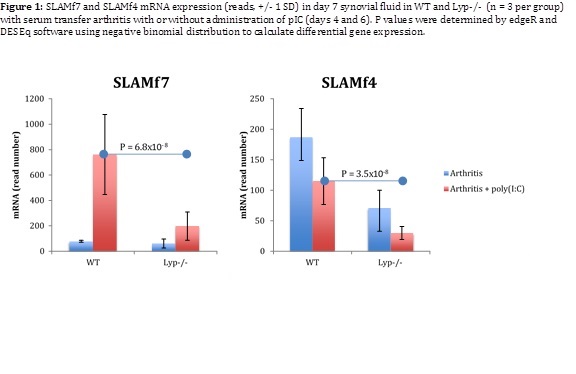
15 genes were differentially-regulated by pIC in Lyp-/- arthritic synovium. Among the genes with known immune-modulating function, Signaling lymphocytic activation molecular family member 7 [Slamf7] and family member 4 [Slamf4] had the highest fold-change differences between genotypes. pIC treatment robustly upregulated expression of Slamf7 in synovium from arthritic WT mice, but upregulation was markedly attenuated in Lyp-/- animals (figure 1). In contrast, Slamf4 transcripts were reduced after pIC treatment in both genotypes; Slamf4 suppression was greater in Lyp-/- mice. Because SLAM family members are important for innate immunoregulation, we examined TLR-signaled SLAM expression within candidate SLAM-expressing innate immune cells. Preliminary results from studies of TLR3 or TLR4-treated bone marrow-derived macrophages or dendritic cells, or of splenic macrophages after in vivo pIC exposure revealed minimal upregulation of Slamf7, regardless of Lyp genotype. NK cells from pIC-treated animals showed upregulation of surface SLAMf7, and suppression of SLAMf4, but not in a Lyp-dependent manner.
Conclusion:
TLR agonist treatment induced modulation of SLAMf4 and 7 and is differentially regulated by Lyp during serum transfer arthritis. Preliminary studies do not support the hypothesis that Lyp modulates TLR-stimulated SLAM family receptor expression in key inflammation-regulating innate immune cell types, including dendritic cells, macrophages, or NK cells.
To cite this abstract in AMA style:
Ewart D, Abrahante Lloréns J, Peterson EJ. Ptpn22 Regulates Synovial Slam Family Receptor Expression during Toll-like Receptor-Driven Suppression of Inflammatory Arthritis [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/ptpn22-regulates-synovial-slam-family-receptor-expression-during-toll-like-receptor-driven-suppression-of-inflammatory-arthritis/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/ptpn22-regulates-synovial-slam-family-receptor-expression-during-toll-like-receptor-driven-suppression-of-inflammatory-arthritis/