Session Information
Date: Tuesday, October 28, 2025
Title: (1780–1808) Osteoarthritis & Joint Biology – Basic Science Poster
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Rheumatic diseases are characterized by pain and a progressive loss of joint function. Classical treatments alleviate pain symptoms but fail to fully repair sites of articular cartilage damage. Gene therapy for rheumatic diseases aims to deliver gene-based treatments into the joint space, resulting in a localized, controlled, and long-term therapeutic effect to protect and regenerate damaged cartilage. Recombinant adeno-associated viral (rAAV) vectors are the only class of vectors currently available that allow for both an effective, direct, and prolonged gene transfer to articular cartilage in vivo. This study aims to characterize the proteomic changes induced by gene therapy on human mesenchymal stromal cells (MSCs) derived from patients with osteoarthritis (OA). The goal is to gain insights into the underlying pathways and mechanisms involved, and to explore their potential roles in cartilage regeneration
Methods: Different chondrogenic factors (IGF-I, FGF-2, TGF-β, SOX9, and reporter lacZ as the control) were delivered using rAAV vectors for hMSC-based gene therapy. hMSC cultures were transduced with the rAAV and maintained for 21 days in a defined chondrogenic medium. Proteomic profiling of the micromasses was performed using high resolution mass spectrometry (TimsTOF-Pro, Bruker). Differential expression analysis was conducted using protein-wise linear models combined with empirical Bayes statistics (adjusted p-value < 0.05 and log2 fold change ≥0.5)
Results: Proteomic analysis was performed on 25 micromass samples (five samples for each chondrogenic factor and control). A total of 3,565 proteins (with >1 unique peptides) were identified in the micromass proteome (Fig. 1). Subsequent data analysis revealed 139, 100, 150, and 230 proteins that were significantly differentially expressed under IGF-I, FGF-2, TGF-β, and SOX9 conditions, respectively. The growth factors IGF-I, FGF-2, and TGF-β shared 16 significant proteins related to metabolic processes and intracellular protein transport (Fig. 2). IGF-I and TGF-β shared 25 proteins related to RHO GTPase effectors and asparagine N-linked glycosylation. IGF-I and FGF-2 shared 22 proteins related to carbohydrate catabolism and translation, while FGF-2 and TGF-β shared 12 proteins involved in membrane trafficking. SOX9 exhibited minimal overlap with the other factors and had 219 uniquely significant proteins associated with ossification, neutrophil degranulation, and extracellular matrix (ECM) components such as glycoproteins, collagens, and proteoglycans. TGF-β modulated 93 exclusive proteins linked to VEGFA-VEGFR2 signalling and proteolysis. IGF-I uniquely affected 71 proteins involved in intracellular EGF-EGFR signalling and metabolism. Finally, FGF-2 was characterized by 45 unique proteins associated with ECM glycoproteins and lipid metabolism
Conclusion: This study provides valuable data supporting the use of gene therapy with chondrogenic factors as a promising strategy for cartilage regeneration. The identification of key proteins involved in cartilage repair and regeneration pathways highlights the therapeutic potential of this approach for the treatment of articular cartilage diseases
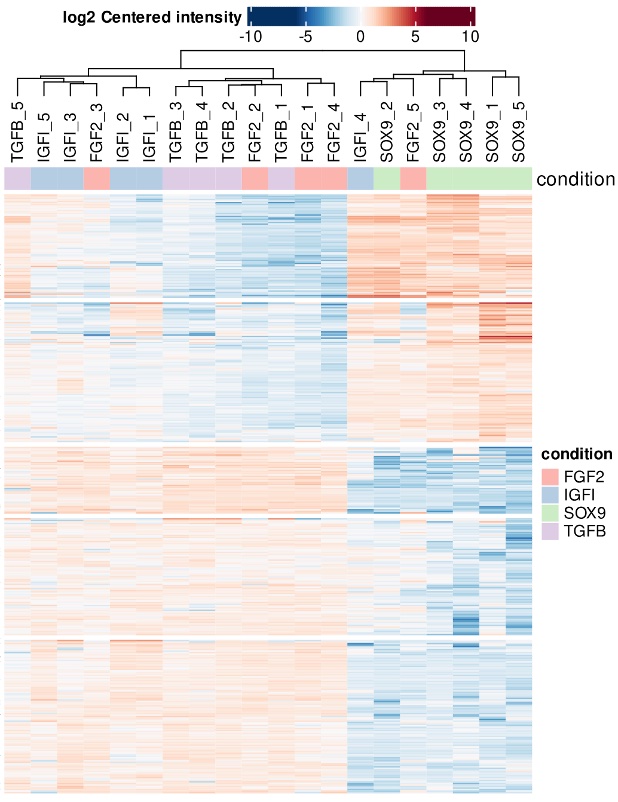 Figure 1. Heatmap showing the differential proteomic profiles induced in hMSCs following gene therapy treatment with various chondrogenic factors. Five independent experiments are displayed, with lacZ used as the control.
Figure 1. Heatmap showing the differential proteomic profiles induced in hMSCs following gene therapy treatment with various chondrogenic factors. Five independent experiments are displayed, with lacZ used as the control.
.jpg) Figure 2. Venn diagram of significant proteins, illustrating the number of unique and shared proteins altered in response to each chondrogenic factor.
Figure 2. Venn diagram of significant proteins, illustrating the number of unique and shared proteins altered in response to each chondrogenic factor.
To cite this abstract in AMA style:
Quaranta P, Venkatesan J, Calamia V, Fernández-Puente P, Blanco f, Cucchiarini M, Ruiz-Romero C. Proteomic Profiling of Chondrogenic Gene Therapy in Human MSCs Reveals Distinct Regenerative Pathways for Articular Cartilage Repair [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/proteomic-profiling-of-chondrogenic-gene-therapy-in-human-mscs-reveals-distinct-regenerative-pathways-for-articular-cartilage-repair/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/proteomic-profiling-of-chondrogenic-gene-therapy-in-human-mscs-reveals-distinct-regenerative-pathways-for-articular-cartilage-repair/
