Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Extracellular vesicles (EVs) have been implicated in autoimmune disease pathogenesis. Plasma-derived DNA containing EVs have been shown to induce STING-mediated proinflammatory responses in dermatomyositis (DM), but their protein content is not well characterized (Li Y et al. Plasma-derived DNA containing-extracellular vesicles induce STING-mediated proinflammatory responses in dermatomyositis. Theranostics. 2021 May 21;11(15):7144-7158).
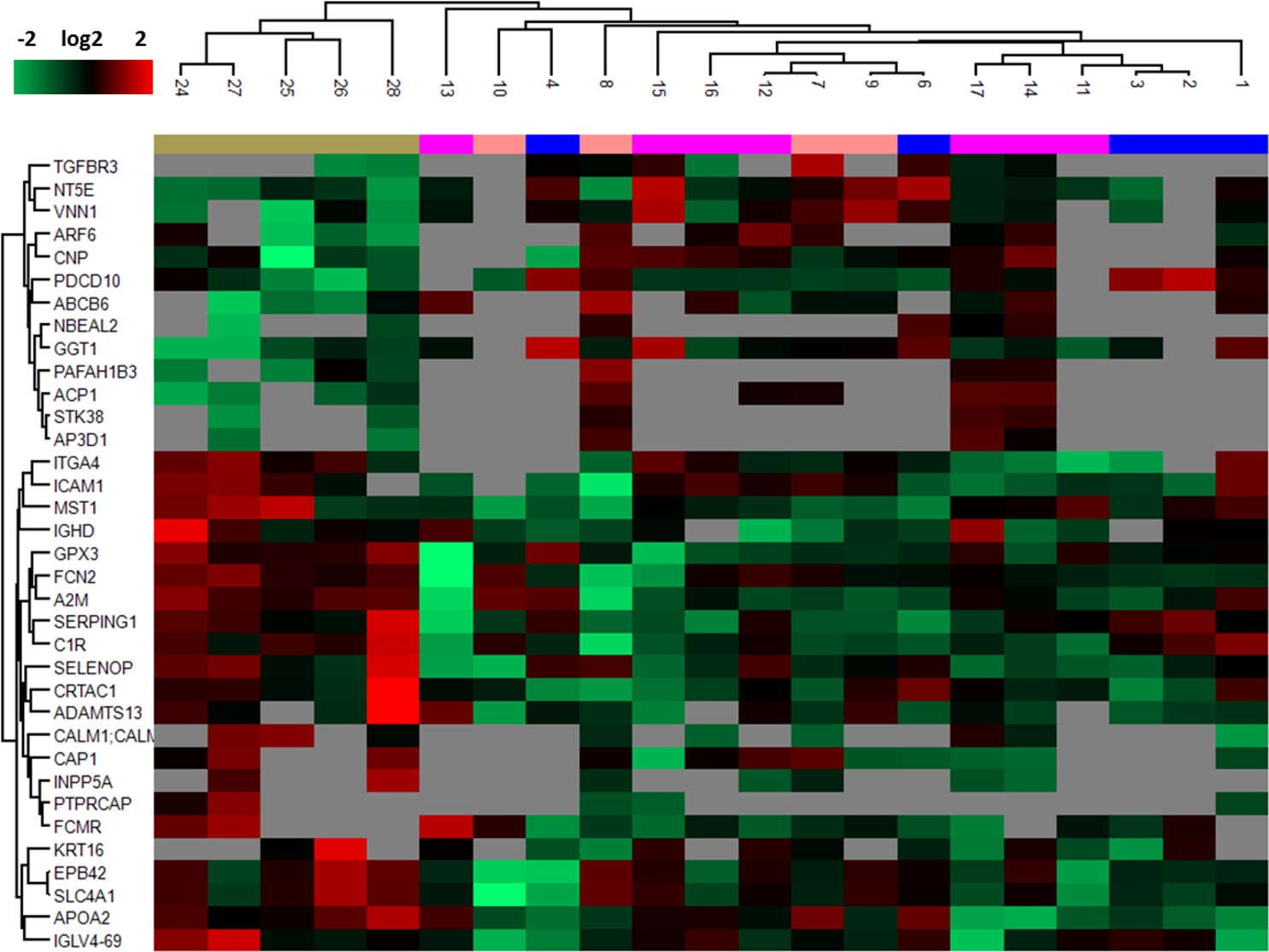
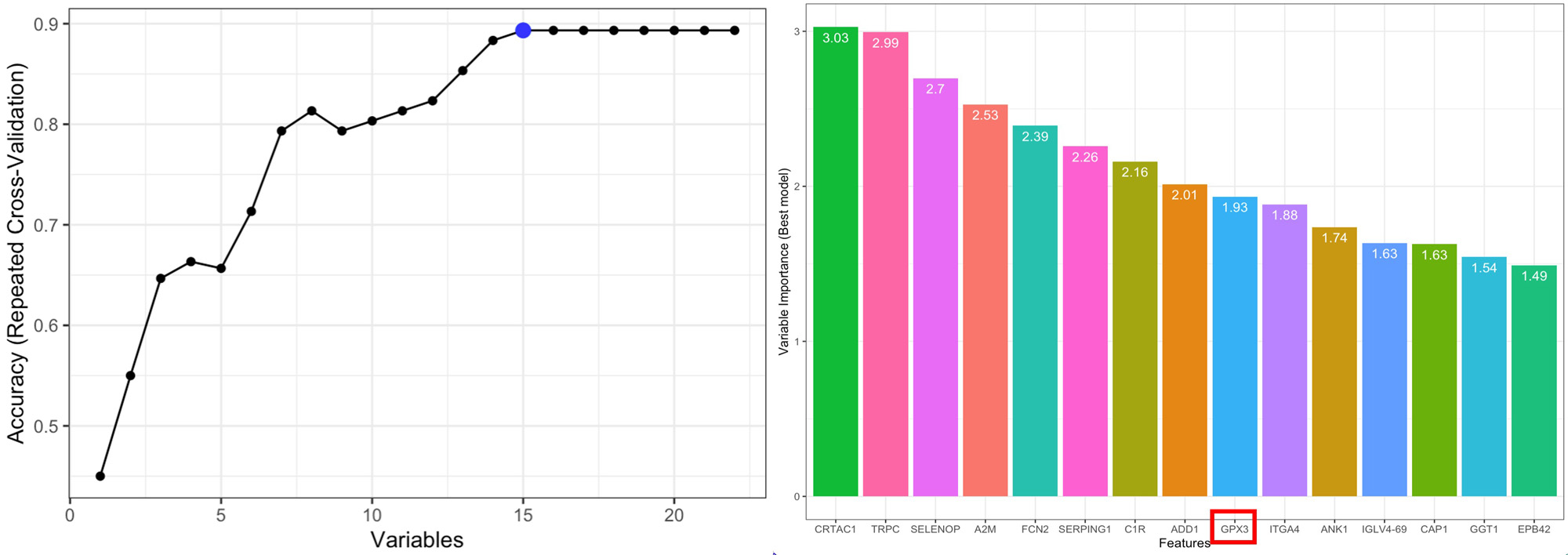
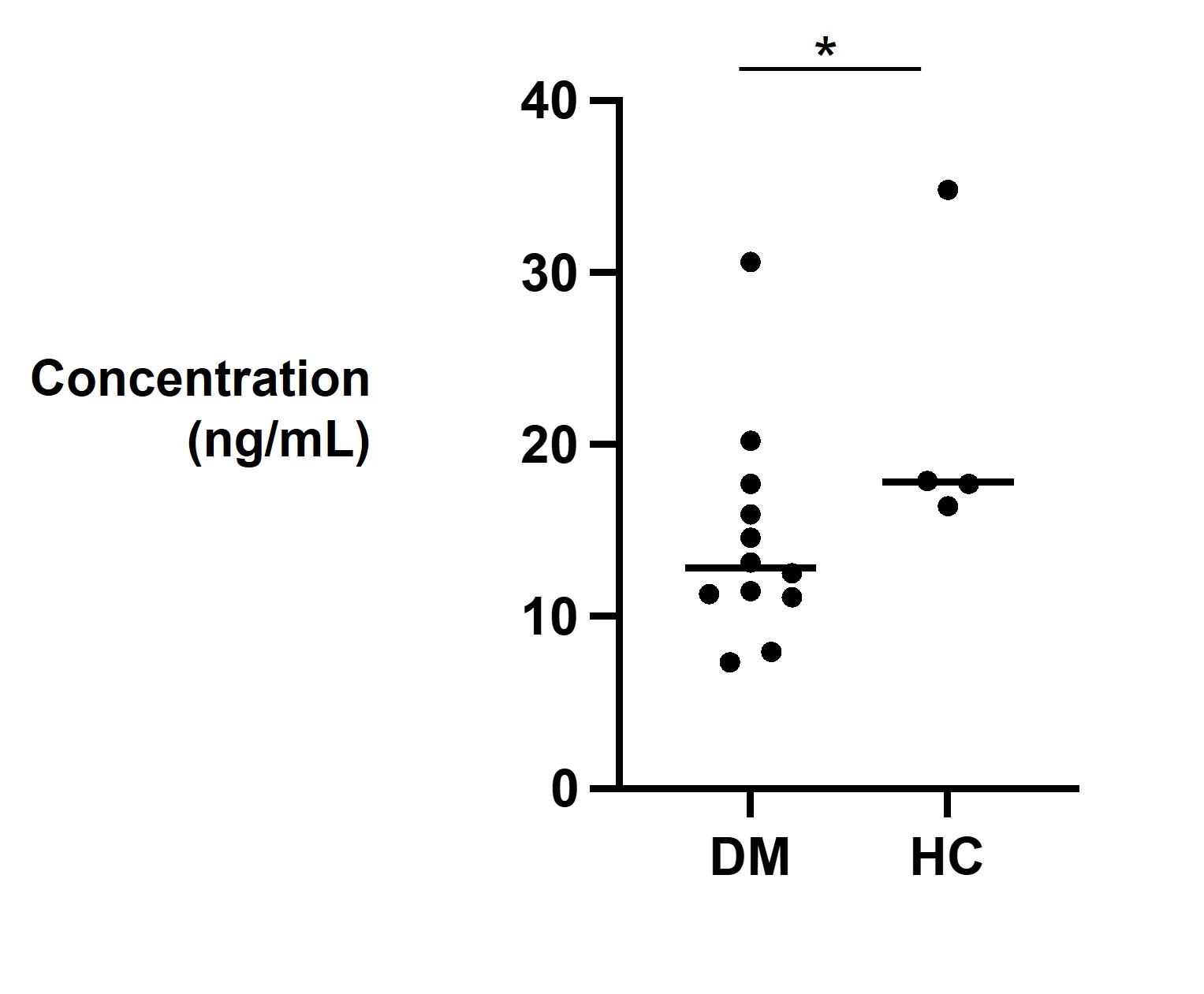
Methods: We collected EVs from plasma of 16 DM patients and 5 controls. EVs were isolated using sequential ultracentrifugation and size- exclusion chromatography, and their content analyzed by mass spectrometry (LC-MS/MS in DIA mode). Over-representation analysis was conducted via http://webgestalt.org based on KEGG,GO and Reactome databases. Protein biomarkers that distinguish patients with DM from controls were assessed using the random forest algorithm. GPX3 abundance was validated in a second cohort by ELISA and data was analyzed by the Mann Whitney test.
Results: Sixty-seven proteins were uniquely detected in the patient cohort. Thirty-five proteins were significantly differentially expressed, of which 13 were upregulated and 22 downregulated. Over-representation analysis found unique and upregulated proteins enriched for myeloid mediated immunity, glutathione metabolism, nucleic acid synthesis and vesicle transport pathways. Downregulated proteins were enriched for the classical and lectin complement pathways. The diminution of complement components in vesicles may reflect its abundance in target tissues but may also reflect host inability to circulate these molecules to damaged tissue, which in a chronic stage of disease may have a protective role. The antioxidant enzyme glutathione peroxidase 3 (GPX3) was significantly less abundant in patients’ EVs compared to controls (p = 0.04) and was assessed by machine learning to be amongst a group of proteins that distinguish patients from controls. GPX3 is a well-known biomarker of certain types of cancer, where its downregulation is correlated with higher inflammatory burden and increased cell proliferation (Nirgude S, Choudhary B. Insights into the role of GPX3, a highly efficient plasma antioxidant, in cancer. Biochem Pharmacol. 2021 Feb;184:114365). A similar mechanism may apply to DM patients, that may be susceptible to oxidative stress due to defective transport of GPX3 by EVs. Alternatively, its lower abundance in EVs may indicate the exhaustion of GPX3 in chronic inflammation.
Conclusion: Our findings indicate GPX3 as biomarker of disease in DM and underlines the importance of oxidative stress in disease mechanism. This finding, along with a growing body of evidence, suggests roles for EVs as disease biomarkers and prompts further mechanistic studies to elucidate the role of GPX3 transport by EVs not only in plasma, but also in muscle and skin.
To cite this abstract in AMA style:
Baniel A, Ogawa-Momohara M, Bashir M, Pandya R, Kleitsch J, Chin F, Liu M, Werth V. Proteomic Analysis of Plasma-derived Extracellular Vesicles Renders Glutathione Peroxidase 3 a Biomarker for Dermatomyositis [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/proteomic-analysis-of-plasma-derived-extracellular-vesicles-renders-glutathione-peroxidase-3-a-biomarker-for-dermatomyositis/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/proteomic-analysis-of-plasma-derived-extracellular-vesicles-renders-glutathione-peroxidase-3-a-biomarker-for-dermatomyositis/