Session Information
Date: Monday, November 14, 2022
Title: SLE – Diagnosis, Manifestations, and Outcomes Poster III: Outcomes
Session Type: Poster Session D
Session Time: 1:00PM-3:00PM
Background/Purpose: Patients with SLE have doubled their utilization of THA and TKA, yet postoperative complications remain high. However, disease specific measures have not been assessed.
Methods: This prospective pilot study enrolled SLE patients with confirmed ACR/EULAR criteria undergoing THA/TKA and sex-, procedure-, and age-matched OA controls between 3/2021-5/2022. Risk stratification markers (BMI, medications, comorbidities) were collected from all patients. SLE activity and damage were evaluated by a rheumatologist on postoperative day 1 (POD-1); disease activity markers were measured at baseline and 6 weeks. D-dimer was measured at baseline, POD-1, and 6 weeks from all patients. PROMs, including PROMIS-10, HOOS/KOOS, SLE flares, and adverse events (AEs) were assessed at baseline, 6-, 12-, and 24-weeks. Continuous data was analyzed using the Kruskal-Wallis and Mann-Whitney U test; categorial data was analyzed using Fishers-Exact test.
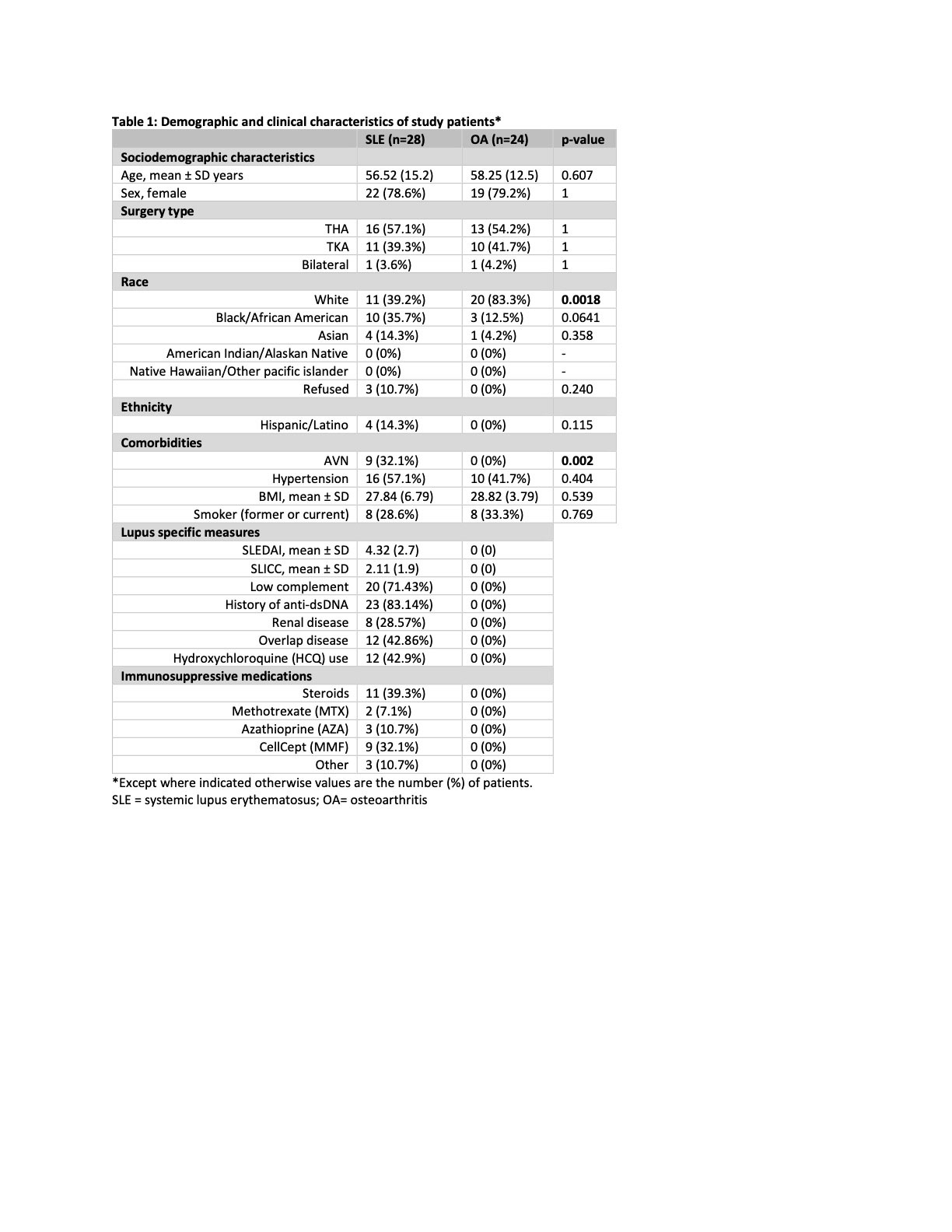
Results: 28 SLE and 24 OA patients were enrolled (Table 1). There were no significant differences in age, sex, ethnicity, or comorbidities between cohorts. A higher percentage of OA patients identified as white (83.3% vs 39.2%, p=0.002). 9 (32%) of SLE patients had evidence of AVN. While the average SLE disease activity index (SLEDAI) was mild (4.32 ± 2.7), many patients exhibited evidence of SLE-related damage, with a mean SLICC of 2.1 ± 1.9.
Baseline D-dimer was significantly higher in SLE than OA patients (0.24±0.08 vs 0.18±0.04, p< 0.001) and increased for both groups postoperatively, remaining elevated at 6 weeks; no DVTs were observed. SLE patients showed significantly lower HgB (12±1.8 vs. 14±0.95, p< 0.001), RBC (4.3±0.66 vs 4.6±0.31, p=0.011), and lymphocytes (1.3 ± 0.57 vs 1.8 ± 0.61, p=0.007). SLE disease activity measures (APS antibodies, C3, C4, ESR, hsCRP, urine sediment) did not change significantly between baseline and 6 weeks.
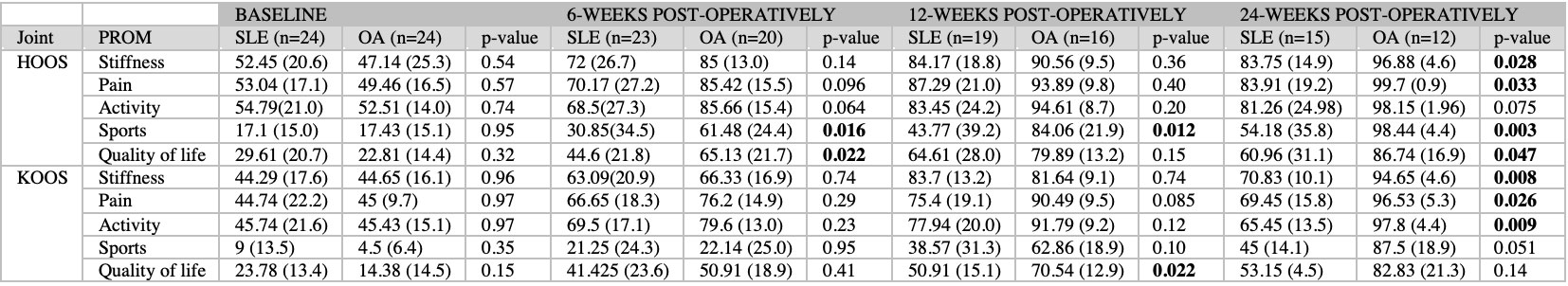
LOS was significantly longer for SLE than OA patients (63 ±37 vs 37 ±17 hr, p=0.004). Including minor events, 16 (57%) SLE patients experienced AEs. (Table 2) SLE patients who experienced AEs had a higher average SLICC-DI than those who did not (2.7 vs 1.3, p=0.02). The majority of SLE patients with AVN experienced an AE (8, 89%). 15 (54%) of patients reported a lupus flare in the 24-week post-operative period. HOOS/KOOS scores showed post-operative improvement in all groups, though the magnitude of improvement was less in SLE than OA patients for all domains. (Table 3) Both mental and physical components of PROMIS-10 showed improvement after THA/TKA, but little change was observed in SLE patients.
Conclusion: Prospective studies of SLE arthroplasty are feasible at our center. The rate of peri- and post-operative complications remains high for SLE patients. The magnitude of functional improvement (HOOS/KOOS) was inferior in SLE patients. A large proportion of SLE patients reported a lupus flare in the 6-month period after surgery. These data may help in understanding physiologic change in SLE and OA patients undergoing THA/TKA and can help in the design of larger studies in this population. We have acquired samples for ongoing biomarker studies to gain further insight into pathophysiologic mechanisms underlying the difference between SLE and OA patients.
To cite this abstract in AMA style:
Fernandez D, Mannstadt I, Figgie M, Sculco P, Blevins J, Siegel C, Jannat-Khah, DrPH, MSPH D, Ford C, Olferiev M, Greenman D, Kirou K, Goodman S. Prospective Study of Clinical, Laboratory, and Patient-Reported Outcomes of Patients with Systemic Lupus Erythematosus Undergoing Total Hip and Total Knee Arthroplasty [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/prospective-study-of-clinical-laboratory-and-patient-reported-outcomes-of-patients-with-systemic-lupus-erythematosus-undergoing-total-hip-and-total-knee-arthroplasty/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/prospective-study-of-clinical-laboratory-and-patient-reported-outcomes-of-patients-with-systemic-lupus-erythematosus-undergoing-total-hip-and-total-knee-arthroplasty/