Session Information
Session Type: Abstract Session
Session Time: 9:45AM-10:00AM
Background/Purpose: Significant changes in gut bacterial richness and diversity occur during the development of inflammatory arthritis, in both murine models and human patients; however, the mechanisms by which dysbiosis promotes disease pathogenesis remain unclear. Because microbiota can shape host physiology through catabolism and production of metabolites, we hypothesized that an altered microbiome during inflammatory arthritis leads to an altered metabalome, which impacts the development of autoimmune B and T cells.
Methods: Cecal metabolites were screened by LC-MS during CIA with antibiotic vs control treatment. CIA was induced by immunization of male 6–8-week-old DBA/1 mice with bovine type II collagen emulsified in complete Freund’s adjuvant at days 0 and 21. To assess how the altered metabolites affected CIA, mice were provided a tryptophan deficient or control amino acid diet starting at day -1 and throughout CIA (with amino acid diet provided on the weekend to compensate weight loss). To supplement indole, 10mM of indole was provided in the drinking water or by oral gavage every other day. To perform the 4-Hydroxy-3-nitrophenylacetyl – chicken gamma globulin (NP-CGG) immunization, NP-CGG was given intraperitoneally (50μg) with 50μg alum adjuvant at days 0 and 10. Finally, to assess direct effects of indole on adaptive immune cells, splenic B cells were evaluated ex vivo after stimulation with LPS and/or anti-IgM for naïve B cells or type II collagen for memory B cell responses.
Results: Products of bacterial catabolism of dietary tryptophan were found to be significantly altered in the cecum of mice. Therefore, mice were given a tryptophan-free diet and found to have significantly decreased CIA associated with an increase in splenic regulatory T cells (Treg). One specific bacterial-derived tryptophan metabolite indole strongly correlated with CIA severity, and direct administration of indole during CIA with antibiotic treatment promoted disease and led to increases in T follicular cell (Tfh) and B cell populations in the Peyer’s patches and mesenteric lymph nodes. Additionally, administration of indole to CIA mice fed a tryptophan deficient diet resulted in greater CIA incidence and splenic Th17 cell frequency. Indole administration was also found to increase splenic activated T cell (CD69+), Tfh, and plama cell numbers in an NP-CGG immunization model. Finally, ex vivo stimulation of B cells was conducted to understand the direct effect of indole, which resulted in significantly increased IgG production and a ≈10% increase in the frequency of CD23+ B cells.
Conclusion: Our results suggest that gut dysbiosis due to CIA results in altered tryptophan metabolism and indole production, promoting CIA pathogenesis via activation of T and B cell populations and antibody production. We hypothesize that the ability of indole to promote Th17 and plasma cell differentiation drives increased CIA development. Precise understanding of which indole metabolites are involved and how they engage cellular receptors will help elucidate the role of intestinal dysbiosis in autoimmunity.
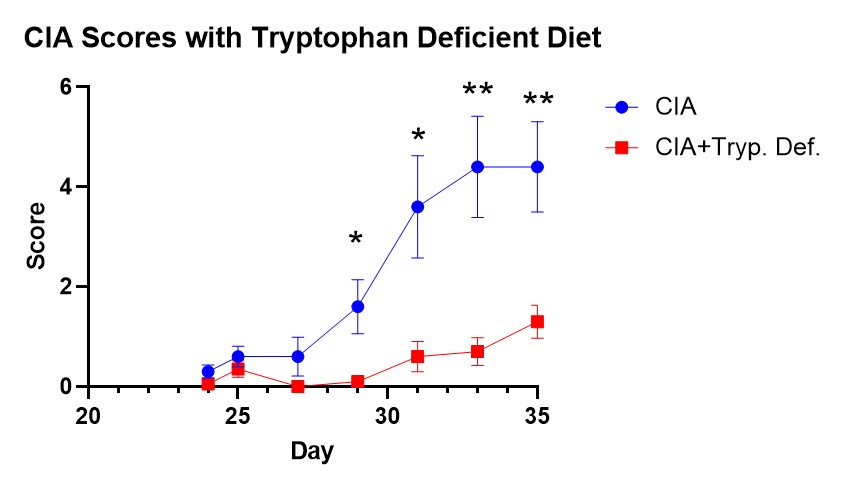 Amelioration of CIA in mice given a tryptophan deficient diet.
Amelioration of CIA in mice given a tryptophan deficient diet.
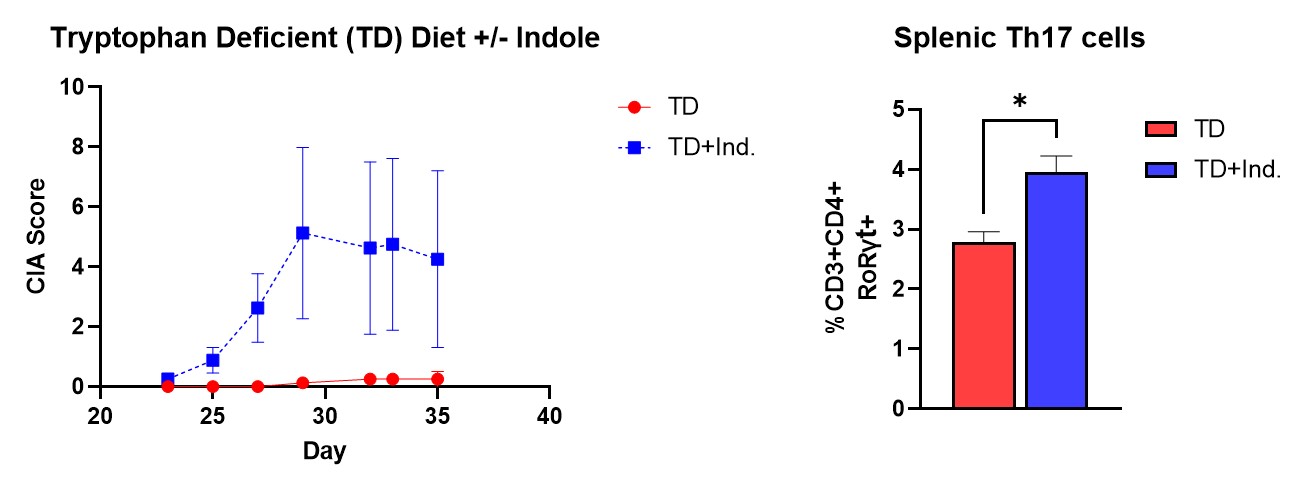 Restoration of a CIA disease phenotype in mice administered the tryptophan bacterial metabolite, indole, during a tryptophan deficient diet.
Restoration of a CIA disease phenotype in mice administered the tryptophan bacterial metabolite, indole, during a tryptophan deficient diet.
To cite this abstract in AMA style:
Trent B, Chriswell M, Jubair W, Kuhn K. Promotion of Autoimmune Arthritis via Tryptophan Metabolism and Production of the Bacterial-Derived Tryptophan Metabolite Indole [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/promotion-of-autoimmune-arthritis-via-tryptophan-metabolism-and-production-of-the-bacterial-derived-tryptophan-metabolite-indole/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/promotion-of-autoimmune-arthritis-via-tryptophan-metabolism-and-production-of-the-bacterial-derived-tryptophan-metabolite-indole/
