Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Nintedanib and pirfenidone are approved therapies for the management of patients with progressive fibrosing interstitial lung diseases (ILD), including those of autoimmune origin (ILD associated with autoimmune rheumatic diseases (ARD) and those with clinical or analytical data suggesting autoimmune origin but not meeting diagnostic criteria).
The objective is to describe the sociodemographic, clinical, functional characteristics, and therapeutic management of patients with progressive fibrosing ILD of autoimmune origin in clinical practice and to evaluate the retention rate of antifibrotic treatment over time.
Methods: Prospective multicenter observational study with inclusion from Jan 2016 to Jan 2024 and follow-up from the start of antifibrotic therapy until discontinuation, loss of follow-up or end of study (May 2024). Subjects: a) ILD associated with ARD, interstitial pneumonitis with autoimmune features (IPAF) or with autoimmune characteristic without meeting diagnostic criteria (UA); diagnosed and seen by a team of pulmonologist and rheumatologist in 8 tertiary hospitals in Madrid; c) with progressive fibrosing ILD; and d) on treatment with nintedanib or pirfenidone. Main variable: antifibrotic drug discontinuation and their causes. Covariates: a) sociodemographic; b) clinical; c) radiological pattern (non-specific interstitial pneumonia [NSIP]; usual interstitial pneumonia [UIP], others); d) FVC % DLCO%; e) laboratory tests; f) therapy used (concomitant corticosteroids, cs/bDMARDs). Source: “NEREA-web registry” (NEumology REumatology Autoimmune). Statistical analysis: A description of the sociodemographic and clinical characteristics of all patients through frequency distribution and the mean and standard deviation or median and percentiles. Survival techniques to estimate the incidence rate of drug discontinuation (IR) expressed in 100 patients-years with their respective 95% CI. Survival will be estimated using Kaplan-Meier curves. Time of exposure comprised the period from the starting date of the antifibrotic until the occurrence of loss of follow-up, main outcome or the end of the study.
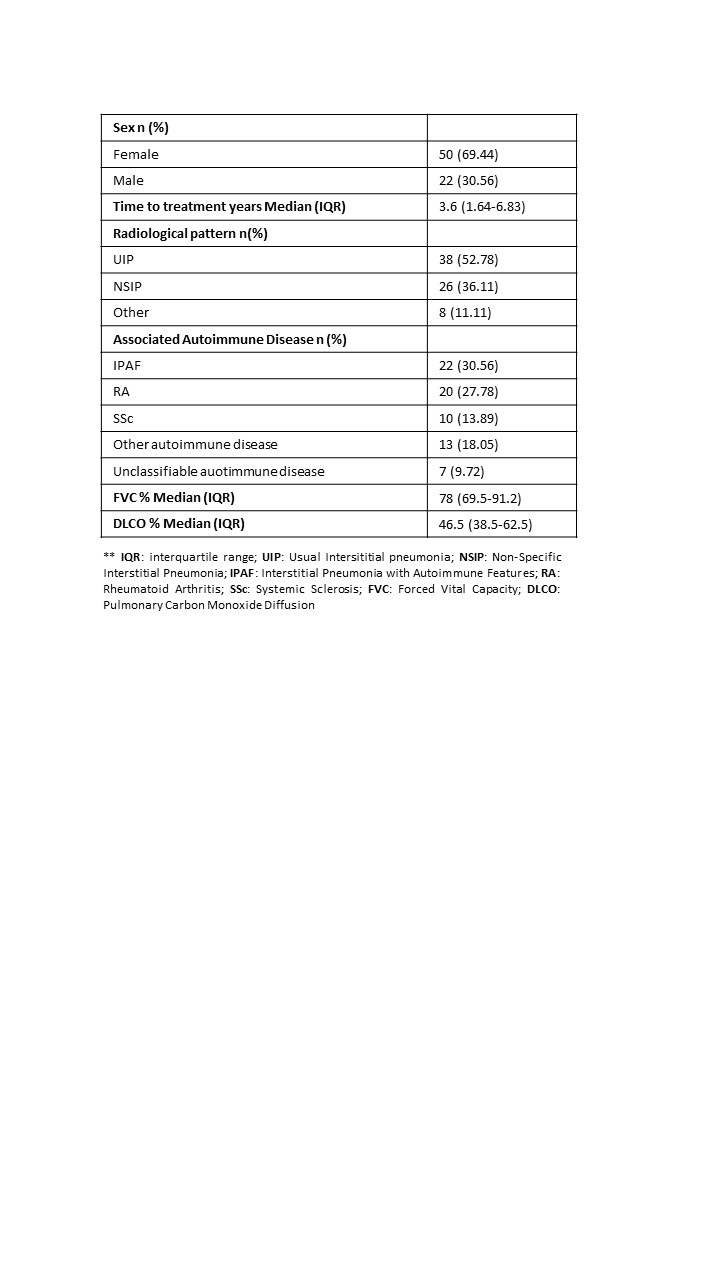
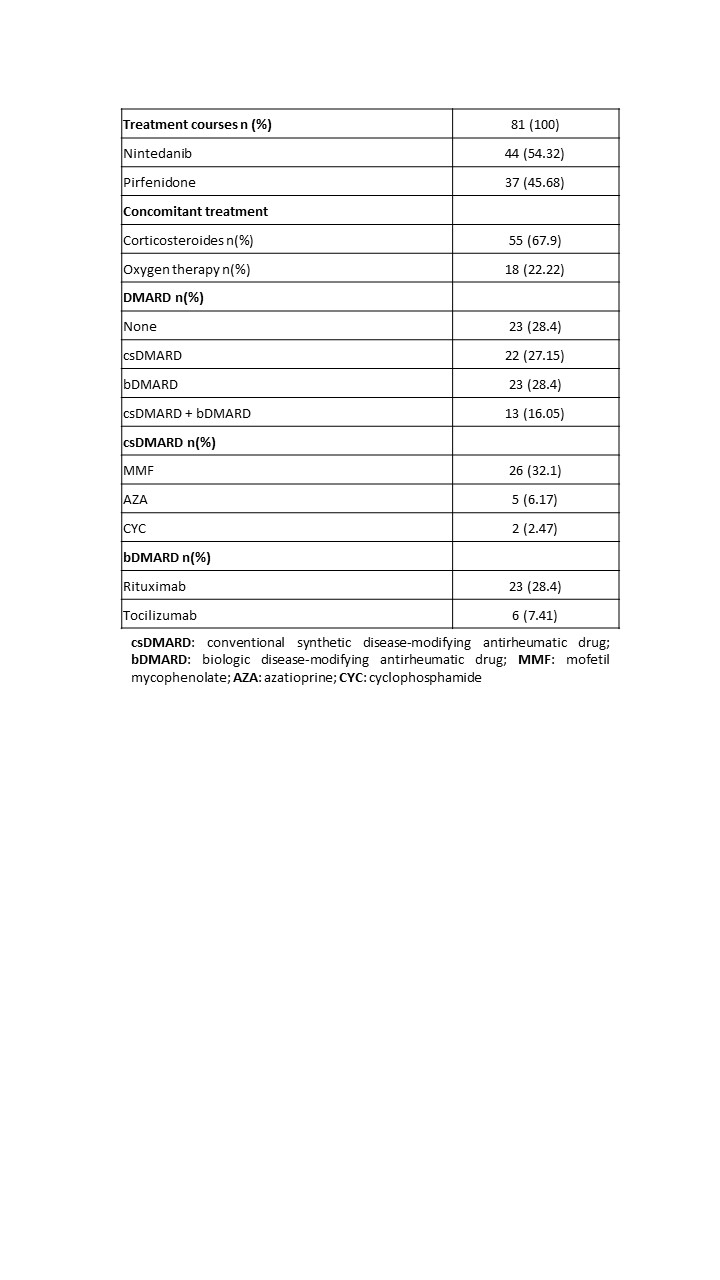
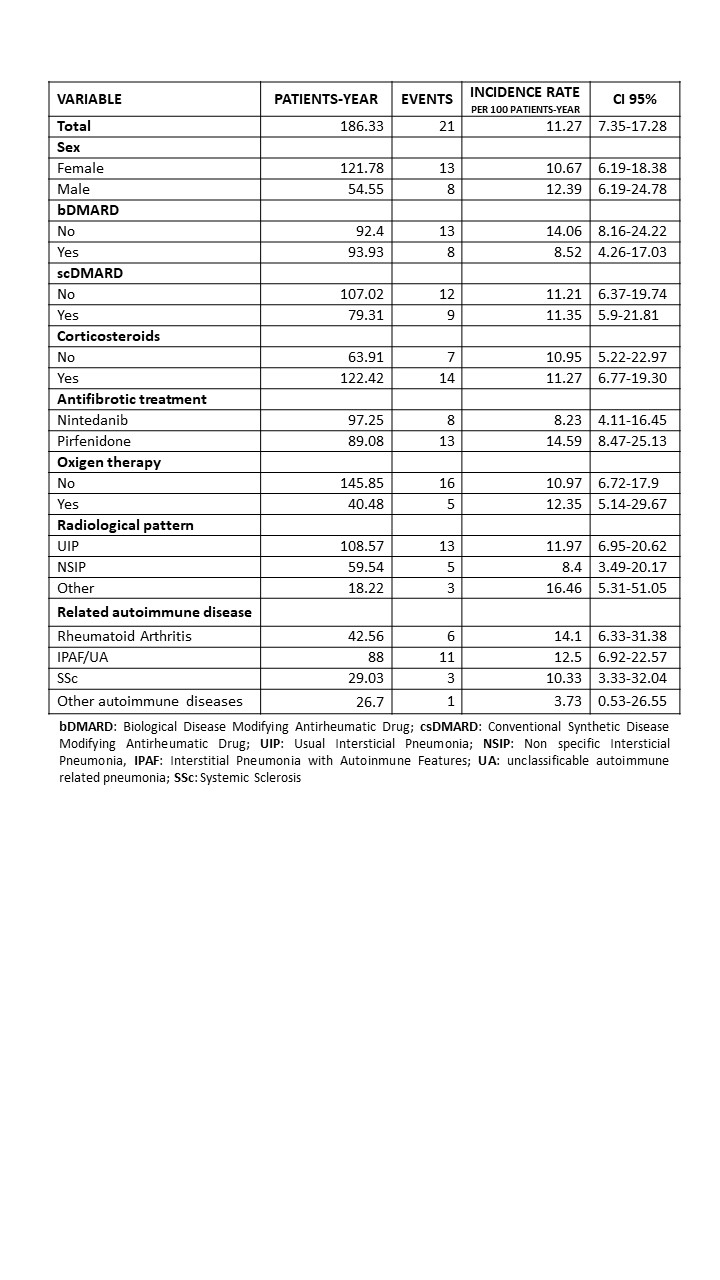
Results: 72 patients (69.44% women) and 81 treatment courses were included with a maximum follow-up of 8 years. The median lag time from ILD diagnosis to treatment start was 3.6 years. Table 1 shows the characteristics of the patients. At antifibrotic start, 71.6% had concomitant DMARD. MMF and rituximab were the most frequently used DMARDs (Table 2). 21 patients discontinued treatment, and the most frequent cause was related to adverse events (n=10, 45%). Discontinuation rate was estimated 11.27, with a median discontinuation time of 5.54 years. Table 3 shows discontinuation rates by variables. Greater persistence of antifibrotics was observed in patients with bDMARDs, NSIP-type radiological pattern, and systemic autoimmune diseases.
Conclusion: Patients with ILD of autoimmunity origin and antifibrotic therapy are mostly IPAF, RA, and SSc, similar to other registries. Most patients at antifibrotic treatment were on concomitant DMARDs. Greater persistence of antifibrotics is observed in patients with bDMARDs, NSIP-type radiological pattern, and systemic autoimmune.
To cite this abstract in AMA style:
Moreno-Fresneda P, Vadillo-Font C, Romero-Bueno F, Sánchez-Pernaute O, Laporta R, Godoy Tundidor h, López-Muñiz B, Cebrián L, Valenzuela C, Llorente-Cubas I, Loarce J, Rigual J, fernandez-Gutierrez B, Nieto M, Abasolo Alcazar l. Progressive Fibrosing Intersticial Lung Disease of Autoimmune Origin and Antifibrotic Medication: NEREA Registry [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/progressive-fibrosing-intersticial-lung-disease-of-autoimmune-origin-and-antifibrotic-medication-nerea-registry/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/progressive-fibrosing-intersticial-lung-disease-of-autoimmune-origin-and-antifibrotic-medication-nerea-registry/