Session Information
Session Type: Abstract Session
Session Time: 1:00PM-2:30PM
Background/Purpose: Rheumatoid arthritis (RA) progression, categorized by EULAR into six stages, includes pre-clinical RA (Pre-RA)[1], where mucosal surfaces are implicated as initiators of autoimmune responses against citrullinated autoantigens[2]. Dysbiosis disrupts immune tolerance, suggesting a “gut-joint axis” for RA intervention[3, 4]. This study therefore explores mixed probiotics’ effects on different stages of arthritis development using the Preclinical phase of collagen-induced arthritis (Pre-CIA) and collagen-induced arthritis (CIA) models.
Methods: DBA/1 mice received collagen immunizations to induce Pre-CIA and CIA models. Both groups received daily oral gavage of a triple probiotic combination (Lactobacillus acidophilus YIT2004, Bifidobacterium longum 6-1, Enterococcus faecalis YIT0072). The Pre-CIA group was assessed on Day 21 and the CIA group on Day 42. Probiotic efficacy was evaluated using clinical and pathological arthritis scores, along with measurement of cytokine levels and T-lymphocyte differentiation. Additionally, 16s rRNA microbiome sequencing and LC-MS-based non-targeted metabolomics were used to explore how gut microbiota changes influence mucosal immunity.
Results:
-
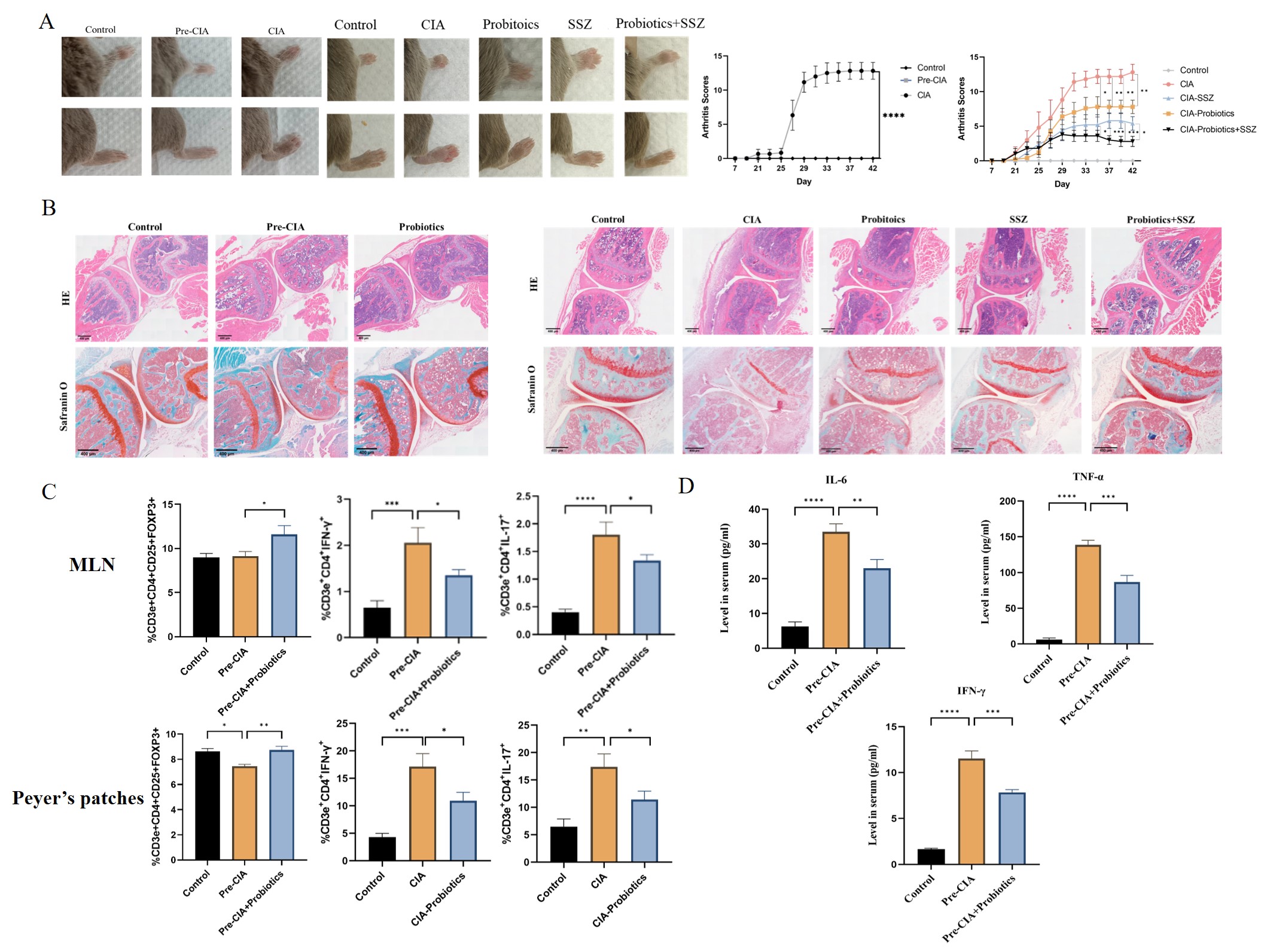
Early intervention with a triple probiotic combination in Pre-CIA phase mice delayed arthritis onset, significantly reduced clinical arthritis scores, and improved pathological manifestations at the CIA endpoint. It primarily regulated T cell levels in gut-associated lymphoid tissue (GALT), markedly increasing Treg cells while decreasing Th1 and Th17 cells, thereby inhibiting IL-6, TNF-α, and IL-17 inflammatory cytokine expressions.
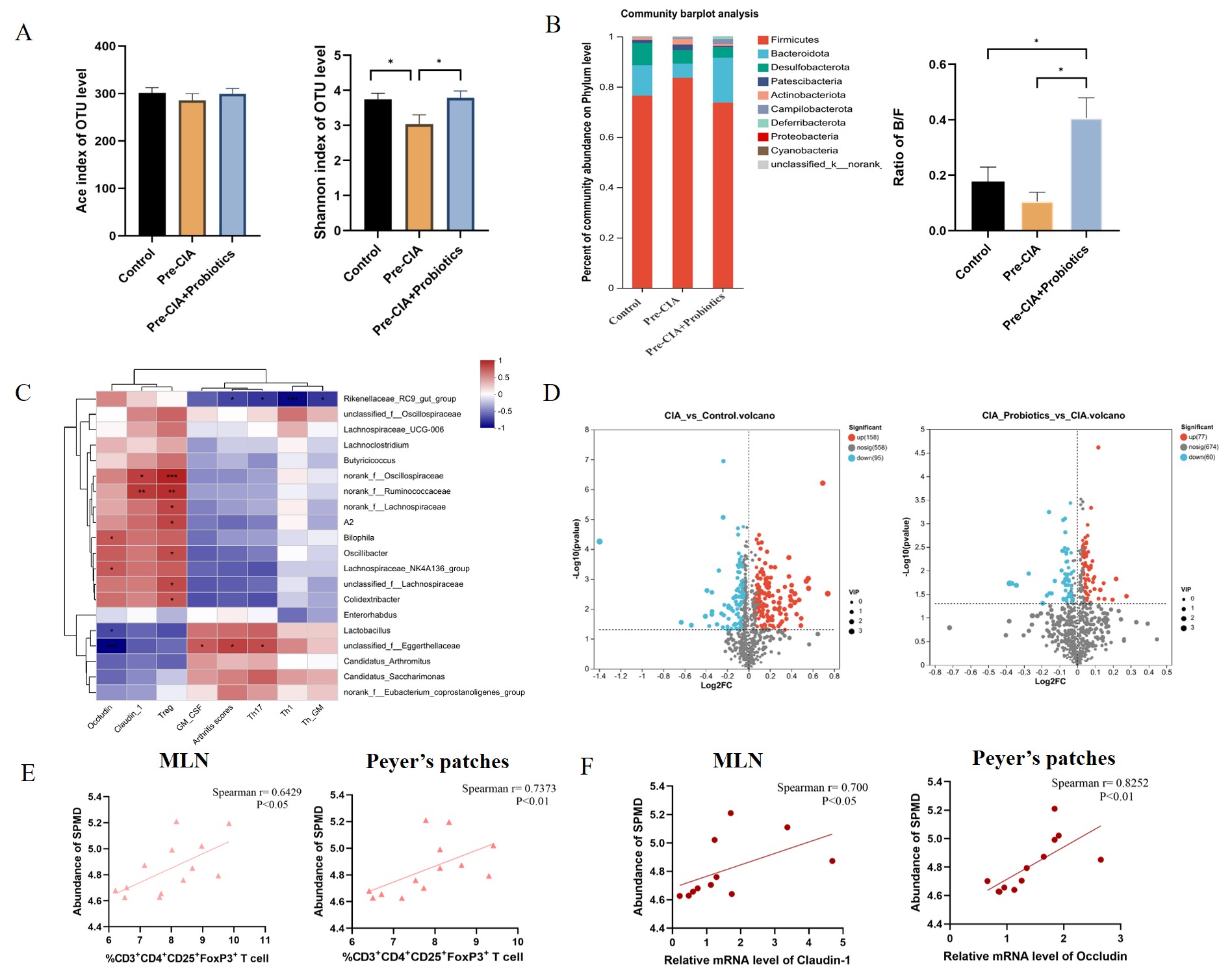
- Probiotic intervention in the Pre-CIA phase modulated gut microbiota, particularly adjusting Bacteroidetes/Firmicutes ratios, and restored decreased relative abundances of Lachnospiraceae_NK4A136_group, etc to normal levels observed in the CIA model. Further correlation analysis between metabolomics and microbiome identified spermidine (SPMD) as a differential metabolite positively correlated with Treg cells and intestinal tight junction proteins.
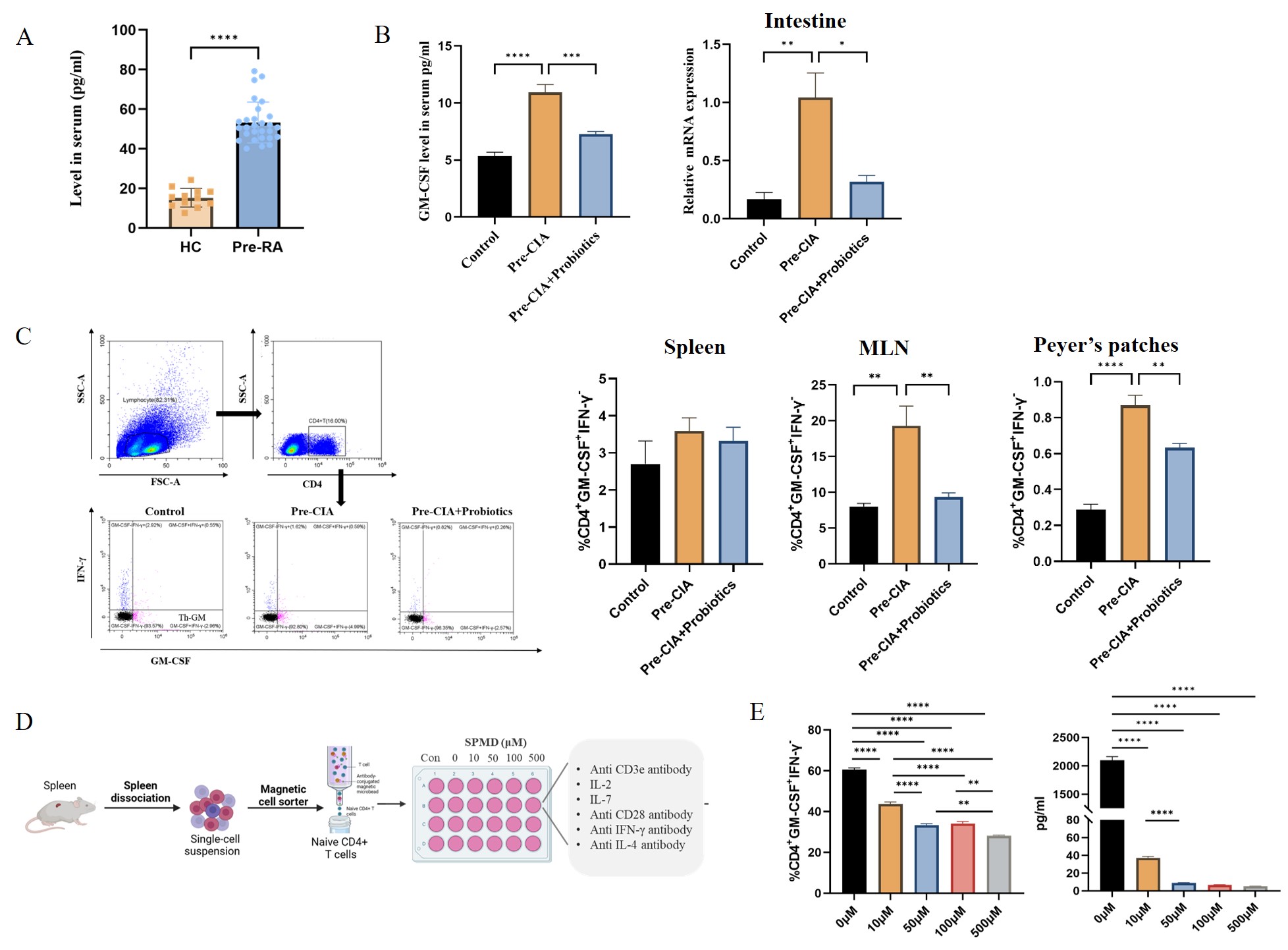
- Elevated serum and intestinal tissue GM-CSF levels were significantly observed in Pre-CIA phase, consistent with findings in Pre-RA populations. Moreover, a notable increase in a novel CD4+ T helper cell subtype (CD4+GM-CSF+IFN-γ- T cell, Th-GM) primarily secreting GM-CSF was detected in GALT. Probiotics downregulated GM-CSF and Th-GM cells, and SPMD inhibited Th-GM differentiation and GM-CSF secretion in vitro (10 μM).
Conclusion: The novel T helper cell subtype Th-GM plays a role in early arthritis via intestinal mucosal immunity, contributing to T cell imbalance. Early supplementation with a triple probiotic regulates gut dysbiosis and enhances gut barrier and mucosal immune responses. This intervention indirectly impacts T cell differentiation, inflammatory cytokines, and immune modulation, delaying arthritis onset and improving clinical outcomes, suggesting probiotics as a non-pharmacological strategy for RA prevention.
To cite this abstract in AMA style:
Wu T, Li Y, Luo Y, Liu Y. Probiotic Modulation of Gut Microbiota Mitigates Early Rheumatoid Arthritis Progression: Insights from Pre-Clinical Models [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/probiotic-modulation-of-gut-microbiota-mitigates-early-rheumatoid-arthritis-progression-insights-from-pre-clinical-models/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/probiotic-modulation-of-gut-microbiota-mitigates-early-rheumatoid-arthritis-progression-insights-from-pre-clinical-models/